Topic: Editing the genes of farmed salmon

Published: 20.08.2019 Updated: 08.02.2022
CRISPR is a technique used to alter the DNA of an organism. There are several ways this can be done: you can inactivate a gene in an organism, modify a gene, or even “paste in” genes from other organisms.
«Breeding» that doesn’t take many generations
Inactivating a gene is the simplest alteration. In many cases, this produces the same results as you could achieve through breeding, only much more quickly. This is the area that marine scientists are particularly focusing on, along with other small modifications to existing genes.
Animals with only minor modifications, and which haven’t received genes from other animals, are also more likely to be approved for human consumption at some point in the future. (See fact box on GMO.)
Have created salmon that could safely escape
Researchers at the Institute of Marine Research (IMR) have successfully inactivated a particular gene in order to prevent farmed salmon from developing germ cells. These are the first salmon in the world without germ cells. As such, they would not be able to breed with wild salmon if they were to escape.
So, although they may still escape, they can’t do as much harm to nature by affecting the genetic make-up of local salmon populations. Moreover, early sexual maturation is a fish welfare issue in fish farming.

There are also other ways to produce sterile farmed fish that don’t involve genome editing, such as triploidisation. Watch a video aabout how researchers produce triploid salmon.
In some cases, triploid fish have had a higher probability of developing skeletal deformities. They are more sensitive to temperature and need special feed. Male triploid salmon may also reach sexual maturity and want to reproduce, even though they are sterile.
These issues mean that researchers are looking for alternative ways of producing sterile farmed salmon.
What about salmon lice?
Escapes and salmon lice are currently the two major sustainability challenges for salmon farming. Is it possible to create a salmon that is immune to salmon lice through genome editing?
Theoretically it might be possible, for instance if a specific gene is responsible for the characteristics that make salmon attractive to salmon lice. If the salmon can cope fine without these characteristics, scientists can eliminate this gene. However, there is a lot of work involved in discovering which genes control which characteristics.

There are Pacific salmon, such as humpback salmon and coho, that are largely resistant to salmon lice. Studying them may offer a good starting point for learning how to make Atlantic salmon more resistant.
Searching for more reliable methods
One important aim of the research on CRISPR and salmon is to develop new methods.
The method currently used on salmon by the marine scientists involves quite a lot of painstaking manual operations. These include injecting special RNA into the egg cell in every individual salmon egg. The special RNA compound, tells the embryonic cells to produce the “scissors” that will specifically cut out or modify candidate genes.
The “scissors” are an enzyme called Cas9. Another RNA substance (a so-called guide) tells the cell which gene to cut out.
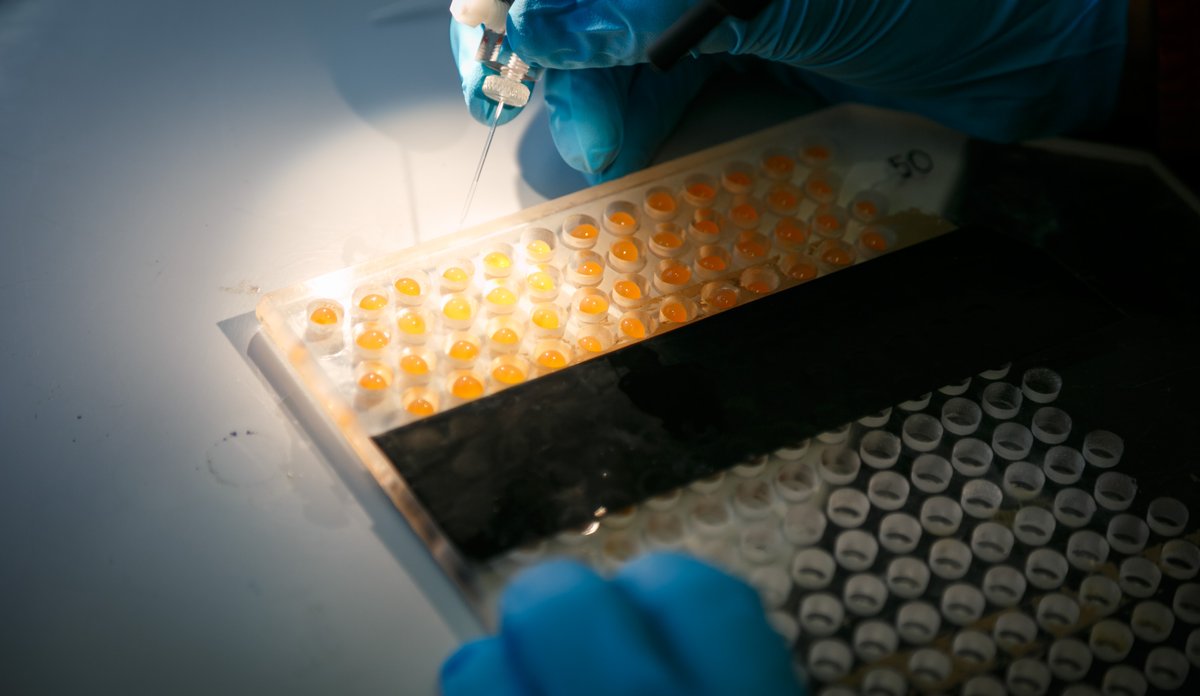
Performing tests on fast-growing fish
In some eggs this works, and the salmon develops without the gene in question. In many eggs, it doesn’t. This could simply be because the scientist has missed with their injection.
Other possible causes include the quality of the various substances used.
The researchers also want to test variants of both the “scissors” and guide to see which one produces the best results.
It greatly helps if you can perform tests on zebrafish first. These small aquarium fish grow much more quickly than salmon.
Salmon only spawn in autumn, and the eggs take three months to hatch. Zebrafish spawn throughout the year, and their eggs hatch within days. This makes trial and error testing much less time-consuming. Learn more about zebrafish: Betting heavily on small tropical fish as experimental species.
As well as working on methodologies, the researchers are continuing to investigate whether genetic engineering can provide solutions to other issues, such as viral diseases.
How it’s done: