Havforskningsinstituttet har undersøkt utbredelsen av infeksiøs lakseanemi virus (ILAV) og salmonid alfavirus (SAV, PD-virus) infeksjoner i utvandrende vill atlantisk postsmolt fanget i 2020 i tre fjordsystemer lokalisert i tre akvakultur produksjonsområder (PO3, 5, 12). Fisken ble samlet inn som en del av NALO-programmet i de ytre delene av fjordene; Hardangerfjorden (N = 100), Romsdalsfjorden (N = 100) og Altafjorden (N = 100) ved tråling i perioden mai-juni. SAV ble påvist i én postsmolt fra Hardangerfjorden og ILAV i én smolt fra Altafjorden. Høy Ct-verdi (37,6) i den SAV-positive fisken kan imidlertid tyde på et falskt positivt resultat. ILAV påvisningen var sannsynligvis av den apatogene HPR0-varianten av viruset. Funnene fra den nåværende rapporten indikerer en svært lav forekomst av disse virusene i vill migrerende postsmolt av laks. Disse funnene utfyller og støtter våre tidligere rapporterte data, og kan antyde at utbredelsen av ILAV- eller SAV-infeksjoner i villaks postsmolt ikke er signifikant påvirket av forekomsten av disse infeksjonene i fiskeoppdrett.
Annual report on health monitoring of wild anadromous salmonids in Norway 2021
— Screening of Atlantic salmon (Salmo salar) postsmolts for viral infections
Report series:
Rapport fra havforskningen 2022-6
ISSN: 1893-4536
Overvåkingsgruppens rapporter
Published: 21.03.2022
Project No.: 15697-01
On request by: Norwegian Food Safety Authority
Research group(s):
Smittespredning og sykdom
Program:
Miljøeffekter av akvakultur
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Terje Svåsand
Norsk sammendrag
Summary
The Institute of Marine Research has investigated the prevalence of infectious salmon anaemia virus (ISAV) and salmonid alphavirus (SAV, PD-virus) infections in migrating wild Atlantic salmon postsmolts captured in 2020 in three fjord systems located in three aquaculture production areas (PO3, 5, 12). The fish were collected as part of the national salmon lice monitoring program in the outer parts of Hardangerfjord (N = 100), Romsdalsfjord (N = 100) and Altafjord (N = 100) by trawling during the period May-June. SAV was detected in one postsmolts from Hardangerfjord and ISAV in one smolt from Altafjord. The Ct-value of SAV-positive fish was however very high (37.6), which may suggest a false positive result. ISAV detected in the positive fish is likely to be avirulent HPR0 variant. The findings from the current report indicate a very low prevalence of these viruses in wild migrating postsmolts. These findings complement and corroborate our previously reported data and may suggest that prevalence of ISAV or SAV infections in wild salmon postsmolts are not significantly influenced by the occurrence of these infections in fish farming.
1 - Introduction
Viral diseases in Atlantic salmon farming in Norway have a negative impact on welfare of infected salmon and often leads to substantial economic losses. The most common viral diseases in salmon farming are pancreas disease (PD), caused by salmonid alphavirus (SAV), heart and skeletal muscle inflammation (HSMI), caused by piscine orthoreovirus 1 (PRV1), cardiomyopathy syndrome (CMS), caused by piscine myocarditis virus (PMCV), infectious salmon anaemia (ISA), caused by ISA virus (ISAV) and infectious pancreatic necrosis (IPN), caused by IPN virus (IPNV).
PD is one of the major diseases in fish farming and much of the fish in endemic areas are believed to become infected with SAV through a production cycle. In Norway, SAV3 was the only subtype detected in salmon farming for a long time, but in 2010 SAV2 was also detected in salmon and since then, PD outbreaks have been dominated by SAV2 in central Norway and SAV3 in western Norway [1].
ISA is a serious disease that has led to severe epizootics in aquaculture in Faroe Islands, Norway and Chile, with enormous economic consequences for industry. Since the 1993, there have been relatively few ISA outbreaks in Norway. However, an increase in the number of ISA outbreaks in recent years along the entire Norwegian coast indicates a growing problem in the salmon farming and an increasing risk of the spread of infection between farms and from infected farms to wild fish. There are two variants of the virus, one that causes disease (virulent, HPR-del) and one that does not cause disease (avirulent, HPR-0). There are growing evidence that virulent ISAV arises from avirulent ISAV, but what triggers this is not yet known [2-4].
Both PD and ISA are notifiable diseases and available data on outbreaks provide a good basis for assessing the possibility for the spread of infection from fish farming to wild salmonids in the sea phase (Table 1).
Table 1: The number of registered PD and ISA outbreaks in fish farming in the years 2016-2021 [1]
|
|
2017 |
2018 |
2019 |
2020 |
2021 |
|
PD |
176 |
163 |
152 |
158 |
101* |
|
ISA |
14 |
13 |
10 |
23 |
25 |
* 82 of the PD-cases are confirmed
Pathogen exchange between farmed and wild salmon occurs, e.g. salmon lice. Hence, disease outbreaks in salmon farms may lead to increased infection pressure on wild fish populations. There is an increasing public concern of this negatively impacting wild salmonids in Norway. However, although the amount of data on the prevalence of virus infections in wild salmonid populations in Norway is increasing [5], the data and knowlegde is limited. Furthermore, it is difficult to quantify disease incidence and its impact in wild fish since sick individuals may be less catchable or may disappear in nature unnoticed (e.g. due to predation). Therefore, it is challenging to evaluate the impact of pathogens on individuals as well as stocks, since we normally are only able to collect infected but non-diseased fish such as individuals that has recently acquired or has survived an infection (carriers). To increase our knowledge, long term surveillance programmes creating a timeseries with sufficient data are necessary.
The effect of fish farming on the infection status of wild salmon stocks may be evaluated by comparing pathogen prevalence in wild fish populations originating from areas having different fish farming intensities and disease outbreak profiles.
Wild salmon may be infected by viruses prevalent in salmon farming; in rivers as fry or parr by virus-infected farmed escapees and spawning wild salmon, or from salmon farms in the fjord when migrating as smolts or returning as adults. Therefore, infection status in migrating smolts may represent a direct indicator of infection pressure from salmon farming during their migration routes. However, the study of viral infection during all life stages of salmon life cycle is necessary to assess overall impact of diseases in fish farming on the salmon wild stocks.
Since 2012, the Institute of Marine Research (IMR) has been commissioned by the Norwegian Food Safety Authority (NFSA) to carry out an annual health monitoring of wild anadromous salmonids in Norway. The current monitoring activities are financed by both NFSA and the Norwegian Ministry of Trade, Industry and Fisheries (NFD). The activities lie within a prioritized research area at IMR which addresses the environmental impact of disease transmission from Norwegian fish farming to wild fish. The surveillance activities aim to evaluate the virus transmission from farmed fish to wild salmonids by monitoring and identifying changes in the prevalence of selected viruses in wild salmonids as a result of fish farming activities. In addition, the surveillance aims to increase the knowledgebase about pathogens in wild salmonids in general, as well as establish a biobank that can be used when new disease challenges arise. Furthermore, the surveillance consolidates with the other activities in the larger strategic research effort on diseases and disease transmission in wild fish.
Part of the research activities in the surveillance program aims to generate data about:
- Virus prevalence in fry, parr, postsmolt and returning adult salmon,
- Prevalence of viruses in sea trout
- Prevalence of infectious escaped farmed salmonids
- Genotypes and characteristics of detected viruses
The virus screening is based on selected materials obtained through monitoring of virus infections in wild salmonids project and other associated projects at IMR, such as:
- National salmon lice monitoring program (NALO)
- National escaped salmon monitoring program
- Etne research station (fish trap)
- Dale research project
- Atlantic salmon at sea (Seasalar) project
- Kolarctic II
The current monitoring program aims to investigate the occurrence of virus infections in wild salmonids captured from different Norwegian coastal areas with different farming intensities and disease outbreak frequencies. Each year selected sets of fish are analysed in order to complement or complete our data and time series. Part of the results from pathogen screening are used in an annual health monitoring of wild anadromous salmonids in Norway commissioned by NFSA. The generated knowledge from the program contributes to the institute's main goal/strategy in providing advice and further development of sustainable management of aquaculture and is utilized in the IMR's annual risk assessment of Norwegian fish farming.
For 2021, the OK program investigated the occurrence of ISAV and SAV infections in migrating postsmolt from the Hardangerfjorden, Romsdalsfjorden and Altafjorden. The three selected fjords are located in three different salmon production areas (PO) that have different farming intensities and disease profiles (Table 2) and were therefore selected based on an assessment of the risk of infection by these two viruses [5]. A hundred fish from each site were analyzed for SAV and ISAV infections using real-time RT-PCR. In total, 600 analyzes has been performed.
Table 2: Fjords (sites) chosen for monitoring of virus infection in postsmolt.
|
Fjord (site) |
Production area |
Farm number* |
PD** |
ISA*** |
|
Hardangerfjorden |
PO3 |
198 |
57 |
2 |
|
Romsdalsfjorden |
PO5 |
67 |
25 |
3 |
|
Altafjorden |
PO12 |
75 |
0 |
12 |
*: Number of fish farms in the production area, **: Mainly caused by SAV3 in PO3 and SAV2 in PO5, ***: Number of ISA cases in 2020 (both suspected and confirmed)
2 - Aim
The aim of the current study was to investigate the occurrence of SAV and ISAV infections in migrating wild Atlantic salmon postsmolts captured in 2020 in three fjord systems located in three aquaculture production areas (PO3, PO5 and PO12) with differing risk for virus infection of wild fish. The numbers fish farms and PD and ISA cases in these areas in 2020 is shown in Table 2.
3 - Materials and methods
To provide data about the prevalence of different viruses in different salmon life stages and different geographical regions, we selected fish from our available material from 2020 for the analysis. These materials were migrating postsmolts captured, as part of the national salmon lice monitoring program [6], in the outer parts of the Hardangerfjorden, Romsdalsfjorden and Altafjorden fjords by trawling during the period May-June 2020 (Fig. 1 and Table 2).
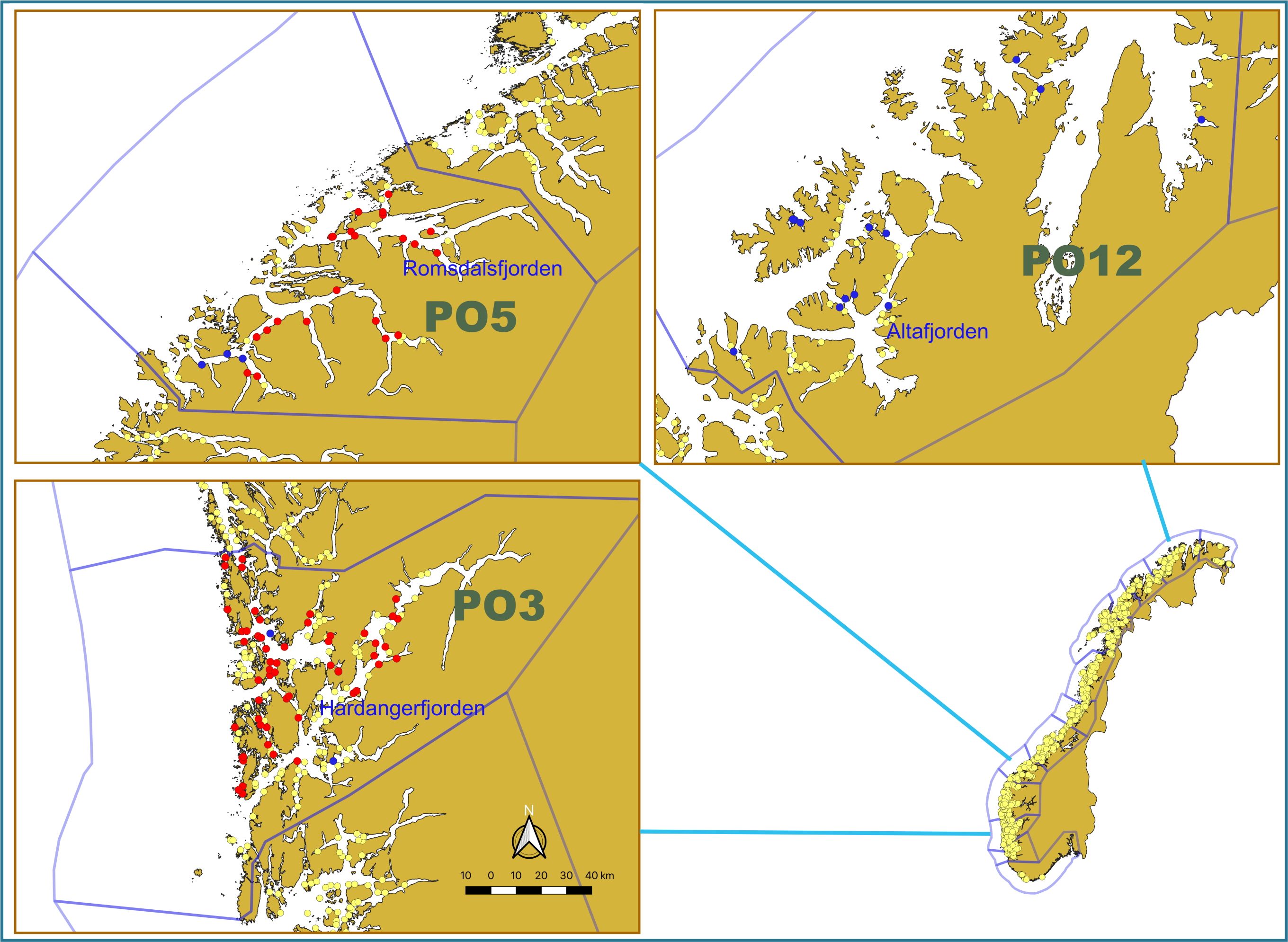
Weight and length of all postsmolt were recorded and the fish were then frozen (-20 oC) as soon as possible. At autopsy, tissues from the gills, head kidney and heart were taken from the fish while still frozen and stored at -80 oC. Samples for analysis were sent on dry ice to an accredited commercial laboratory for RNA extraction and virus testing (Pharmaq Analytiq AS; https://www.pharmaq.com/en/analytiq). All fish were tested for SAV and ISAV (Table 2) by real-time RT-PCR assays (for detection viral RNA). A total of 600 analyses were performed on 300 fish and included in the current report.
4 - Results
ISAV was detected in one postsmolts from Altafjorden (Ct-value 31.5) and SAV in one postsmolt from Hardangerfjorden (Ct-value 37.6) (Table 2).
5 - Discussion and Conclusion
ISAV was detected in one migrating postsmolt from production area PO12. The high Ct-value (31.5) of PCR may indicate a low virus concentration. The virus variant (HPR-0 or HPR-del) could not be verified as it was not possible to sequence the virus due to the low viral-RNA concentration in the positive sample. However, the virus was detected in gills but not in heart of the postsmolt, something that may indicate that the variant is HPR-0 as this virus variant infects gills but not heart [2].
There were 12 ISA cases in the collection area during the postsmolt migration period (Fig 1) and the exposure to virulent ISAV (HPR-del) has therefore been considered moderate [5]. However, it is unlikely that the virus detected in the positive fish was originated from these ISA outbreaks as these are caused by the virulent HPR-del variant. On the other hand, avirulent ISAV (HPR-0) is prevalent in Norwegian salmon farming [3] and was also detected in wild salmon populations collected in other areas in Norway [7, 8]. Our recent study showed that ISAV (HPR-0) was detected in 7% of wild returning adult salmon from northern Norway [8]. The results from this study and our monitoring program showed that ISAV (HPR-0) infection in wild salmon may occur and that this occurrence is not necessarily associated with salmon farming [5, 8]. Additionally, these results showed no significant difference in the prevalence of ISAV‐HPR0 between the wild and the escaped farmed fish. This finding is interesting as previous reports have shown that escaped farmed fish are more frequently virus‐infected than wild salmon [9-11]. This can be explained by the transient nature of ISAV‐HPR0 infection in salmon which may limit the detection‐time window of the virus [2].
Unlike ISA, PD is prevalent (endemic) in two of the postsmolt collection areas (PO3 and PO5). Therefore, it is likely that migrating postsmolt were exposed to SAV released from the fish farms. However, SAV was detected in very low concentration (Ct-value 37.6) in only one postsmolt from Hardangerfjorden. The Ct-value is just above the detection limit of the PCR assay (cut-off 37) and may suggest a false positive. The low prevalence of SAV infections in the tested migrating smolt is consistent with previous findings in wild salmonids [12-20]. Our earlier report showed that migrating smolts from Trondheim fjord which has no fish farming activities also had very low occurrence of SAV infection [13]. Similarly, very low prevalence of SAV was detected in returning adult salmon, postsmolt at Norwegian sea or juveniles from Norwegian rivers [5].
Our previous and current findings showed no apparent relationship between the prevalence of virus infection in wild salmon and the fish farming intensity or the frequency of disease outbreaks in collection areas [10, 18-20]. These observations may indicate that wild salmon are exposed to a low infection pressure from fish farming. However, the possibility that infection may lead to rapid disappearance or altered behaviour of the infected fish, and therefore may affect the results, cannot be ruled out. Other explanation for the low prevalence of viruses in postsmolt is the time needed after virus exposure (incubation time) before the virus can be detected in tissues of fish. To verify our observation, a large PD-vaccine smolt release study was therefore conducted in 2018 and 2019. In this study, a total of 52 000 (28 000 PD-vaccinated and 24 000 control) smolt were released in rivers Etneelva and Daleelva located in production areas (PO3 and PO4) which had a high number of PD cases during the release period. The survival rate of returning adult salmon in the subsequent years were determined in both vaccine and control groups and used to estimate the mortality that may be attributed to SAV infection from fish farming in release areas. The current results (unpublished) from the study did not show any statistically significant differences in the mortality rate between the vaccine and the control groups and therefore support our observations that infection pressure of SAV from fish farms to wild salmon is low. Furthermore, screening smolt used in sentential cages placed in PO3 for a period of 2 weeks for ISAV and SAV infections did not reveal virus infection in these smolt.
The results in the current report showed very low occurrence of infections in migrating postsmolts in fjords for two viruses occurring in Norwegian aquaculture. These findings complement and corroborate our previously reported data and may suggest that prevalence of ISAV or SAV infections in wild salmon at different life stages are not significantly influenced by the occurrence of these infections in fish farming. It is also suggesting that it is unlikely that wild salmon is a reservoir that spill over these viruses to fish farming. There are still significant gaps in our knowledge about diseases in wild fish and the interaction between farmed and wild fish [5]. Time series of samples of all life stages of wild salmonids from areas with different salmon farming intensities are necessary to better evaluate and understand the long-term effect of infection pressure from aquaculture on the virus prevalence in wild salmon populations. Such series will also enable us to assess changes in the prevalence due to increased fish farming activities, the emergence of new diseases and climate change.
6 - References
- Sommerset, I., et al. (2022). Fish health report 2021 (in Norwegian). Norwegian Veterinary Institute 2a/2022. Available at https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2022/fiskehelserapporten-2021
- Rimstad, E., and Markussen, T. (2020). Infectious salmon anaemia virus-molecular biology and pathogenesis of the infection. Journal of Applied Microbiology, 129:85-97.
- Lyngstad, T.M., et al. (2012). Low virulent infectious salmon anaemia virus (ISAV-HPR0) is prevalent and geographically structured in Norwegian salmon farming. Diseases of Aquatic Organisms, 101:197-206.
- Christiansen, D. H., et al. (2017). First field evidence of the evolution from a non-virulent HPR0 to a virulent HPR-deleted infectious salmon anaemia virus. Journal of General Virology, 98:595-606.
- Grefsrud, E. S., et al. (2021). Risk assessment of Norwegian fish farming. Rapport fra havforskningen 2021-8: Institute of Marine Research, Bergen. Available at https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-2021-8
- Nilsen, R., et al. (2017). Lakselusinfestasjon på vill laksefisk langs norskekysten i 2016; med vekt på modellbasert varsling og tilstandsbekreftelse. Rapport fra Havforskningen 1-2017: Institute of Marine Research, Bergen
- Nylund, A., et al. (2019). Wild and farmed salmon (Salmo solar) as reservoirs for infectious salmon anaemia virus, and the importance of horizontal- and vertical transmission. Plos One, 14(4) doi:ARTN e021547810.1371/journal.pone.0215478
- Madhun, A.S., et al. (2019). Prevalence and genotypes of infectious salmon anaemia virus (ISAV) in returning wild Atlantic salmon (Salmo salar L.) in northern Norway. Journal of Fish Diseases, 42:1217-1221
- Garseth, Å. H., et al. (2013). Associations between piscine reovirus infection and life history traits in wild-caught Atlantic salmon Salmo salar L. in Norway. Preventive Veterinary Medicine, 112:138-146.
- Madhun, A. S., Isachsen, C. H., Omdal, L. M., Bardsgjaere Einen, A. C., Bjørn, P. A., Nilsen, R., and Karlsbakk, E. (2016). Occurrence of salmonid alphavirus (SAV) and piscine orthoreovirus (PRV) infections in wild sea trout Salmo trutta in Norway. Diseases of Aquatic Organisms, 120:109-113.
- Madhun, A. S., et al. (2017). The ecological profile of Atlantic salmon escapees entering a river throughout an entire season: diverse in escape history and genetic background, but frequently virus-infected. Ices Journal of Marine Science, 74:1371-1381.
- Biering, E., et al. (2013). Annual report on health monitoring of wild anadromous salmonids in Norway 2012. Rapport fra Havforskningen 6-2013: Institute of Marine Research, Bergen. 13 pp. Available at http://www.imr.no/filarkiv/2013/03/annual_report_on_health_monitoring_of_wild_anadromous_salmonids_in_norway-rapport_fra_havforskningen_nr._6-2013_.pdf/nb-no
- Madhun, A. S., et al. (2014). Annual report on health monitoring of wild anadromous salmonids in Norway 2013. Rapport fra Havforskningen: Institute of Marine Research, Bergen. 15 pp. Available at https://imr.brage.unit.no/imr-xmlui/handle/11250/280534
- Garseth, Å. H., et al. (2015). Annual report on health monitoring of wild anadromous salmonids in Norway 2014. Rapport fra Havforskningen 7-2015: Institute of Marine Research, Bergen. 14 pp. Available at https://www.imr.no/filarkiv/2015/04/helseovervaking_av_vill_laksefisk_i_norge_2015.pdf/nb-no
- Madhun, A. S., et al. (2016). Annual report on health monitoring of wild anadromous salmonids in Norway 2015. Rapport fra Havforskningen 22-2016: Institute of Marine Research, Bergen. Available at https://www.imr.no/filarkiv/2016/05/hi-vi-health_monitoring_of_anadromous_salmonids-2016-web.pdf/nb-no
- Garseth, Å., et al. (2017). Annual report on health monitoring of wild anadromous salmonids in Norway 2016. Rapport fra Havforskningen 17-2017: Institute of Marine Research. 15 pp. Available at https://imr.brage.unit.no/imr-xmlui/handle/11250/2477613
- Madhun, A. S., et al. (2018). Annual report on health monitoring of wild anadromous salmonids in Norway 2017; Health monitoring of returning adult salmon from river Etne, western Norway. Rapport fra Havforskningen 26-2018: Institute of Marine Research, Bergen. 13 pp. Available at https://www.hi.no/en/hi/nettrapporter/rapport-fra-havforskningen-en-2020-16
- Madhun, A. S., et al. (2019). Annual report on health monitoring of wild anadromous salmonids in Norway 2018; Screening of migrating Atlantic salmon (Salmo salar) postsmolts from the Trondheim fjord for viral infections. Rapport fra Havforskningen 2019-28: Institute of Marine Research, Bergen. 9 pp. Available at https://www.hi.no/en/hi/nettrapporter/rapport-fra-havforskningen-en-2019-28
- Madhun, A. S., et al. (2020). Annual report on health monitoring of wild anadromous salmonids in Norway 2019. Rapport fra Havforskningen 2020-16: Institute of Marine Research, Bergen. Available at https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2020-16
- Madhun, A. S., et al. (2021). Annual report on health monitoring of wild anadromous salmonids in Norway 2020. Rapport fra Havforskningen 2021-19: Institute of Marine Research, Bergen. Available at https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2021-19