Toktrapport for 2025007011 gir en oversikt over aktivitetene som ble gjennomført under Mareanos første tokt til den arktiske midthavsryggen (AMOR) i september og oktober 2025. Arbeidet som ble utført på dette toktet krevde en tilpasning av metodikken i forhold til utfordringene knyttet til prøvetaking og visuelle undersøkelser i dype og heterogene miljøer med kupert terreng som er vanlig på AMOR. Denne rapporten presenterer en oversikt over aktivitetene som ble utført på toktet, detaljerte beskrivelser av de nye metodene som ble brukt, foreløpige analyser av dataene med et første blikk på habitatene og geologiske forhold som forekommer i det undersøkte området, samt anbefalinger for fremtidige Mareano-tokt i området.
MAREANO's first cruise to the Arctic Mid-Ocean Ridge
— 2025007011 Cruise Report
Report series:
Toktrapport 2025-11
ISSN: 1503-6294
Published: 12.12.2025
Cruise no.: 2025007011
Project No.: 16156-02
On request by: MAREANO
Research group(s):
Bunnsamfunn
Program:
Marine prosesser og menneskelig påvirkning
Research group leader(s):
Sigurd Heiberg Espeland (Bunnsamfunn)
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Frode Vikebø
Norsk sammendrag
Summary
The 2025007011 cruise report provides an overview of the activities completed during MAREANO’s first cruise to the Arctic Mid Ocean Ridge (AMOR) in September and October 2025. The work done on this cruise required an adjustment of methodology to fit the needs of working in deep and heterogeneous environments that is common on AMOR. This report records the activities performed on the cruise, detailed descriptions of the new methods used, preliminary analyses of the data with a first look at the habitats and geological setting present in the surveyed region, and recommendations for future MAREANO cruises in the area.
1 - Introduction
MAREANO Cruise 2025007011 is MAREANO’s first cruise to the area that Norway has opened for mineral activity on the Arctic Mid-Ocean Ridges (AMOR). MAREANO is tasked with providing knowledge and baseline ecodiversity information that can address the management needs for the Deep Norwegian Sea both inside and outside of the mineral exploration area through the collection of bathymetric, geological, biological, and chemical data.
AMOR is an ultra-slow spreading ridge located between Greenland and Norway made up of 6 ridge segments - Ægir, Jan Mayen, Kolbeinsey, Mohn’s, Knipovich, and Gakkel Ridge (Pedersen et al., 2021). There are large variations in depth along the spreading axis of AMOR generally ranging between >3500 m to <500 m depth. This is due to the deep rift valleys at the spreading axis extending into the steep mountainous terrain along the axis before turning into deep abyssal plains as one moves farther away from the spreading zone. However, some areas on AMOR can be much shallower or deeper than the average, such as the Seven Sisters Vent Field near Jan Mayen at 130 m depth or Molloy Deep on Knipovich Ridge at 5569 m depth.
Parts of AMOR (particularly along the crest of AMOR in the Norwegian Sea) has also been defined as a particularly vulnerable and valuable areas (SVO-områder: Særlig verdifulle og sårbare områder på norsk; NH4; Meld. St. 21 (2023–2024) - regjeringen.no). This includes the Jan Mayen ridge, Jan Mayen fracture zone, Mohn’s ridge, Knipovich ridge and the Molloy Deep. Parts of the survey boxes on this cruise overlap with this SVO-area.
Due to the great depths and heterogeneous terrain in AMOR that differs from standard MAREANO conditions on the Norwegian Shelf, intensive planning and adjustment of methodology was required for the preparation of the cruise, as suggested in the Deep-Sea Strategy (Ross et al., 2025). In addition to mapping the priority area, a secondary objective of this cruise was to test and determine the effectiveness of the modifications and refine sampling methodology for future cruises along AMOR. Detailed description of the methods used are described below in Section 3. Methodology.
1.1 - Oceanographic Setting
Located between the Greenland, Iceland, and Norwegian (GIN) Seas, AMOR is subjected to a complex oceanographic setting as it forms a boundary between the Greenland and Lofoten Basins. Due to its positioning, AMOR interacts with different water masses on both sides of the ridge system. At the surface (approximately upper 50 m), the cool and low salinity Greenland Polar Water (T < 5°C, S < 34.4 ppt) is brought down from the northwest with the East Greenland Current and the warm and high salinity North Atlantic Water comes up from the southeast with the Norwegian Atlantic Current (T > 2°C, S > 35 ppt) (Hopkins, 1991). Moving down in the water column, transitional water (or intermediate water) forms between the surface waters and the deep waters, where to the west of AMOR, the less dense, cooler and fresher Greenland Arctic Intermediate Water forms (T < 2°C, S ~ 34.7-34.9 ppt), and to the east is Norwegian Arctic Intermediate Water (T ≃ 0.5°C, S ≃ 34.88). Deep water is formed in the GIN Seas basin, where forming in the Greenland Basin, the cooler and fresher Greenland Sea Deep Water forms below 2000 m (T = -1.25°C, S = 34.89), and the Upper Norwegian Deep Water (T = -0.5°C, S = 34.92) and Norwegian Sea Deep Water (T ~ -1.05°C, S ~ 34.91) in the Norwegian Sea above 2500 m and below 2500 m, respectively.
While the surface water is known well in the area (Hopkins et al., 1991; Roberts et al., 2018), there is limited data on the deep and intermediate water masses in the region, particularly in oceanographic models. Water masses are continually reported as important factors that influence the distribution of benthic fauna and respective biotopes (Burgos et al., 2020; Roberts et al., 2021), therefore it is important to collect data from the deeper regions where possible.
1.2 - Station Planning
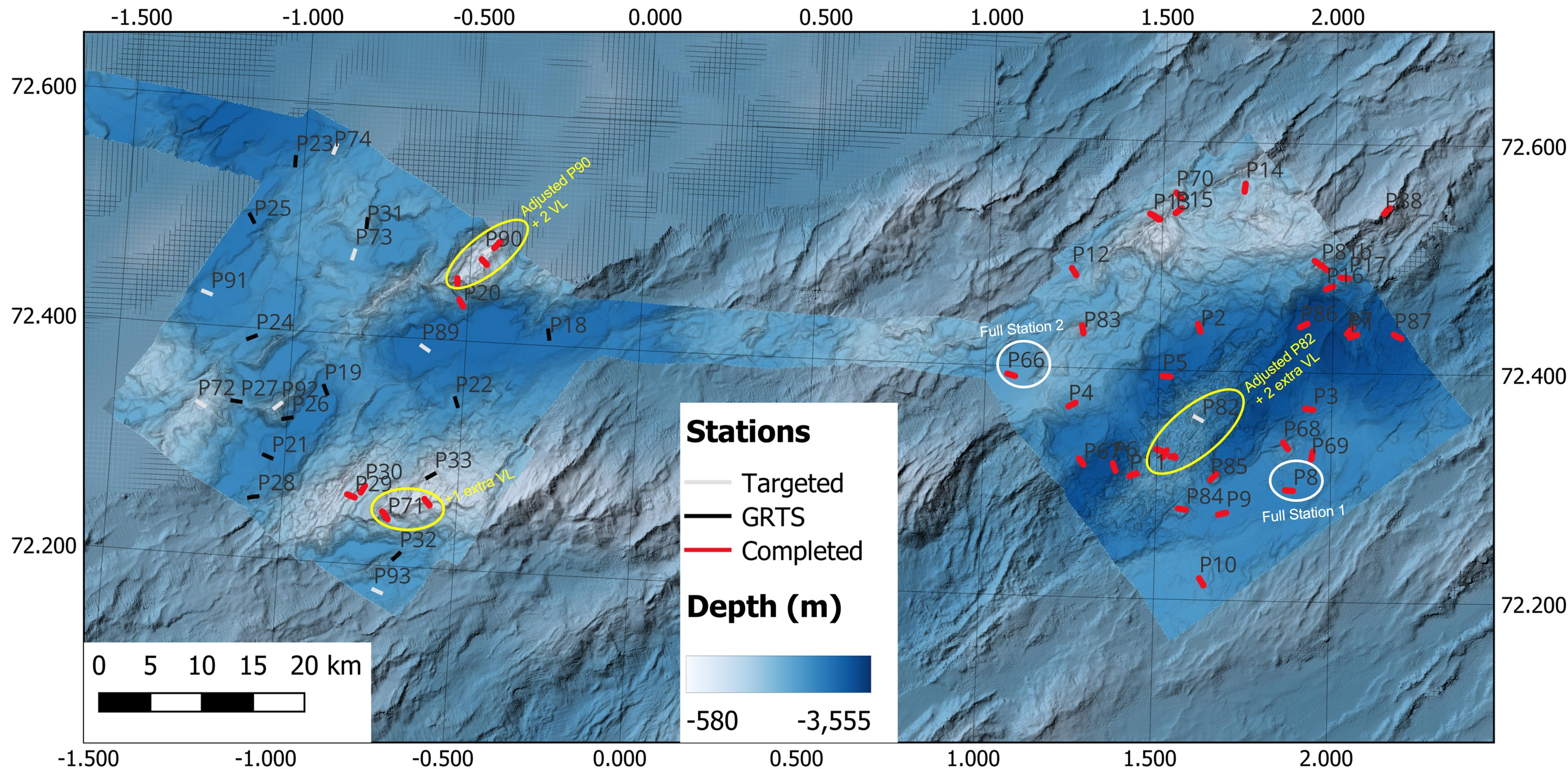
The priority boxes for Cruise 2025007011 were located on Mohn’s Ridge (figure 1) – NH3-B06, NH3-B07, and NH3-B08 with a total of 79 stations with 6 full stations (2 per box). Two additional boxes (NH3-B09 and NH0-B03) with a total of 47 stations with 4 full stations (2 per box) were planned as reserve in case of spare time or weather limited access in the priority areas. Bathymetry would be collected between the boxes during transit.
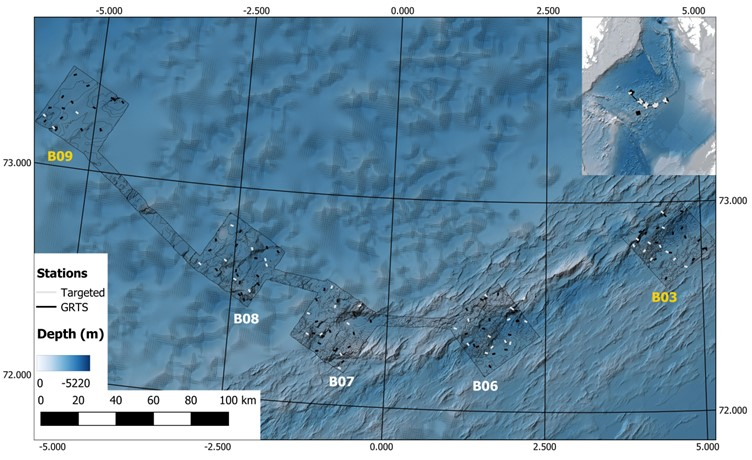
NH3-B06 (approx. 1300 km2) was the first box to be surveyed (Figure 2). It is located directly on the spreading zone and has heterogenous terrain consisting of vent fields (e.g., Ægir Spring), seamounts (e.g., DeepInsight), volcanic mounds, ridges, and rift valleys, with the stations covering a depth range of 1320 to 3340 m, with an average depth of 2500 m. There were 17 GRTS (Generalized Random Tessellation Stratified) and 14 Targeted stations planned, with one of the targeted stations (P88) located just outside of NH3-B06 to investigate a bamboo coral aggregation observed in a survey conducted by the Norwegian Offshore Directorate in 2024. There were 5 potential stations for full stations.

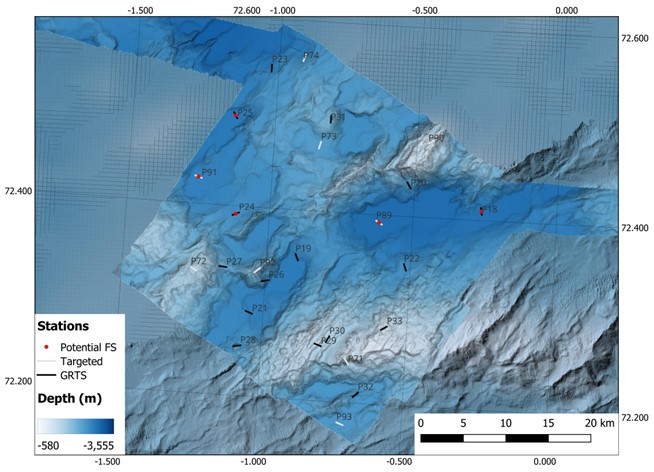
NH3-B07 (approx. 1300 km2) was the second planned box and the stations covered a depth range of 1155 to 3340 m depth (Figure 3). It was located off the main spreading axis and contained heterogeneous terrain consisting of ridges, seamounts, and deep basins. It had 16 GRTS and 8 Targeted stations planned, with 5 potential stations for full stations.
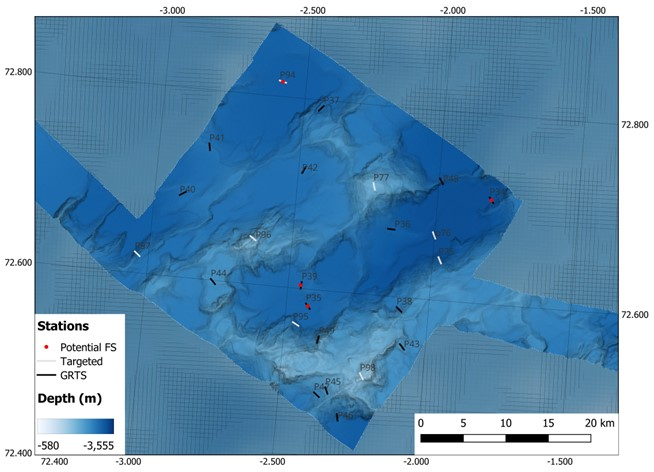
NH3-B08 (approx. 1300 km2) was the third planned box, located off of the spreading axis entirely (Figure 4). It had a depth range of 1850 to 3275 m in fairly homogeneous terrain consisting of small seamounts and deep basins. It had 16 GRTS and 8 Targeted stations planned, with 4 potential full stations.
2 - Cruise Participants
| Name | Institute | Role |
| Heidi Kristina Meyer | HI | Cruise Leader |
| Pål Buhl-Mortensen | HI | Cruise Leader |
| Roy Holger Robertsen | HI | Instrument Chief |
| Fredrik Frigstad | HI | Instrument |
| Kjell Bakkeplass | HI | Data Management |
| Stepan Boitsov | HI | Chemist |
| Èric Jordà Molina | HI | Biologist |
| Ragni Olssøn | HI | Biologist |
| Camille Saint-André | HI | Biologist |
| Heidi Gabrielsen | HI | Biologist |
| Jonatan Fredricson Marquez | HI | Biologist |
| Nils Piechaud | HI | Biologist |
| Irina Zhulay | HI | Biologist |
| Valérie Bellec | NGU | Chief Geologist |
| Lilja Rún Bjarnadóttir | NGU | Geologist |
| Christine Tømmervik Kollsgård | NGU | Geologist |
| Daniel Hesjedal Wiberg | NGU | Geologist |
| Rosalyn Fredriksen | SoDir | Observer |
| Anja Helene Bang | UiB | Master’s Student |
| Björn Löfqvist | ROV | ROV Night Supervisor |
| Andreas Storebø | ROV | ROV Day Supervisor |
| Johan Sköld | ROV | Pilot |
| Hilbert Í Grógv | ROV | Pilot |
| Kenneth Ågotnes Fosse | ROV | Pilot |
| Dánial Johannesen | ROV | Pilot |

3 - Methodology
To effectively survey the deep sea, a variety of modifications were required from the Standard MAREANO survey design for both video lines and full stations (ref. to previous survey design) to be able to use the time most wisely and sample most effectively.
3.1 - Video Lines
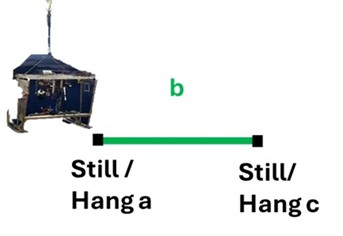
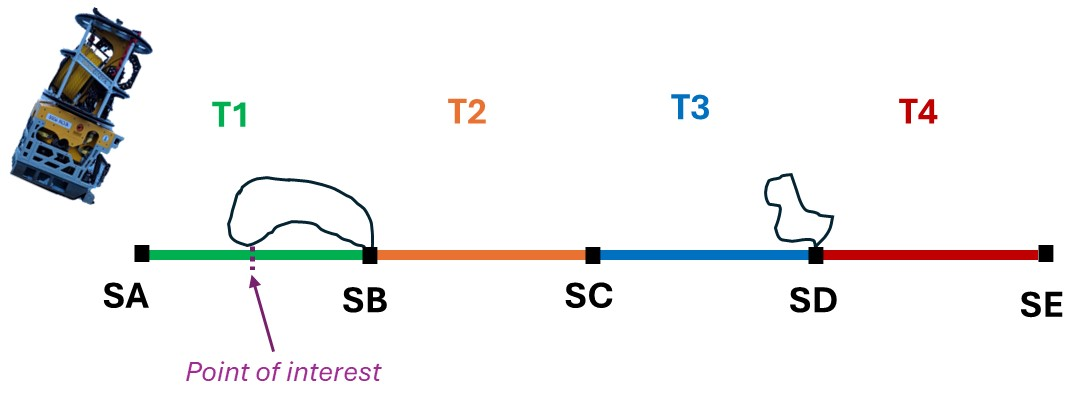
Video lines were adjusted from the standard 200 m to 800 m long, where there would be 4x 200 m long dedicated transects per video line. Sampling and investigation were only possible in sections between the 200 m long transects.
The naming conventions used in the deep-sea video lines were also adjusted where all non-quantitative portions of a video line would be called “Section” and denoted with a letter rather than “Still” or “Hang”, and all quantitative portions of the video line would be called “Transect” and denoted with a number rather than the segment only being referred to as a letter.
During a “Transect”, the ROV would move at a stable speed (approximately 0.4 knots) and altitude (approximately 1 m) without stopping. Any points of interest would be noted and revisited for further investigation or sampling during the following “Section”. Transects are the segment of the videos meant for quantitative analysis post cruise. During a “Section”, the ROV would scan the field of view before investigating, sampling, or moving to waypoints placed during the “Transect”. Sections will not be annotated post cruise; however, they may be used to get clearer identifications of objects or species post cruise .
3.1.1 - Standard MAREANO Video Line Design:
Traditional MAREANO surveys on the Norwegian Shelf typically use the towed camera system, Chimaera, and have 200 m long video lines (Figure 5).
3.1.2 - Deep MAREANO Video Line Design:
Deep-sea MAREANO surveys on AMOR used NORMAR’s remotely operated vehicle (ROV) Ægir6000 and have 800 m long video lines made up of 4x 200 m transects (Figure 6).
3.2 - Remotely Operated Vehicle (ROV)
Due to the difficult terrain and great depths, NORMAR ROV Ægir6000 was used in place of MAREANO’s towed-camera Chimaera for collecting the visual data. Ægir6000 is a work-class ROV manufactured by Kystdesign AS (Haugesund, Norway) that has a depth rating of 6000 m water depth. Its dimensions are 2.75 x 1.70 x 2.20 m with the tool skid and has a load capacity of 350 kg. It has a hydraulic drawer mounted on the skid, with a mountable suction sampler. It has two Imenco Spinner II (HD) cameras mounted on the top and center of the ROV. Two green Manta Ray mk2 Parallel Lasers are mounted directly on top of the center camera and spaced 9 cm apart. It has two manipulator arms: TITAN 4 with an arm camera, LED light and a lift capacity of 122 kg; and ATLAS which has a lift capacity of 250 kg. Ægir6000 is attached to the Tethered Management System (TMS) to improve stability and operability when operating at large depths.
In addition, geological, biological, and chemical samples were collected in situ during designated sections along the video line with the use of sampling gear designed for Ægir6000. Due to the lack of knowledge of the biology in the area and the difficulties in accessing the deep sea, it is important that MAREANO collects biological samples during video lines to detect diversity that otherwise would be missed in the video for identification and to achieve higher taxonomic resolution than is possible from video alone, and as a supplement to the physical fauna collection.
During a standard dive, not a full station, the following gear were mounted onto the ROV (Photo 2):
-
2 plastic push corers on the drawer
-
8 plastic push corers mounted on the TMS
-
2 mesh nets (1 long and 1 short)
-
1 “Frankenstein” scoop
-
1 suction sampler (with 5 chambers)
-
2 toolboxes (for biological/geological samples)
-
1 “biobox” (for biological/geological samples)
-
1 knife

Additional gear that was brought for specific dives in targeted stations or stations with specific conditions were:
-
2 niskin bottles (for eDNA or water samples)
-
1 temperature probe
-
1 major sampler (for water samples)
-
2 blade corers with the transparent plexiglass plates (for biological samples)
Additional gear that was brought for full stations were:
-
2 niskin bottles (for eDNA)
-
2 blade corers with aluminum plates (for chemistry)
-
2 aluminum push corers mounted on the TMS (in place of 2 plastic push corers; for chemistry)
3.2.1 - ROV Geological Sampling
Sediment push cores (up to 40 cm long and 9 cm in diameter) were retrieved for geology at the beginning and/or end of the video lines, and during sections if the seabed was suspected to have changed. Photos were taken during sampling to see the area it was retrieved from, and the coordinates and depth noted down. When the samples were on board, photos were taken of the outside before opening (Photo 3), of the core after opening and of the core split in two. Then the core was logged by MAREANO standards and hand samples taken for future evaluation of the grain size and archive, before everything was entered into NGUs sample description logs in Survey123.

Physical rock samples were retrieved using the ROV-Arm or the ‘Frankenstein’ scoop. Photos were taken during sampling to see the area it was retrieved from, and the coordinates and depth noted down. The samples were also assigned an event number (see further description in Section 3.2.2. ROV Biological Sampling). When the samples were on board, they were briefly described in hand-written paper logs and photo-documented (Photo 4). New Survey123 sample description logs will be designed for rock samples before the next cruise to the area.

An attempt was made to measure the temperature inside an active chimney with a temperature probe, but this was unsuccessful as it did not record it due to its logging time being shorter than the manual said.
3.2.2 - ROV Biological Sampling
Biological samples were taken with the ROV Ægir6000 with a wide variety of tools (Figure 7; see Section 3.2. Remotely Operated Vehicle (ROV)). The sampling was aimed at improving the taxonomic resolution of species observed in the video footage. Each time the ROV sampled an item (biology, geology, and chemistry) with either a new tool or different location, the sample was assigned a rolling “Event ID” number and logged as a comment in Seabed Field Observer (SFO; described further in Section 3.3. Seabed Field Observer). A photo of the screen was captured during the sampling event to help identify the sample once Ægir6000 was on deck. In a physical log, the Event ID was recorded with the sampling gear type, the storage compartment on the ROV and a “video name” describing what the targeted specimen(s) or object(s).
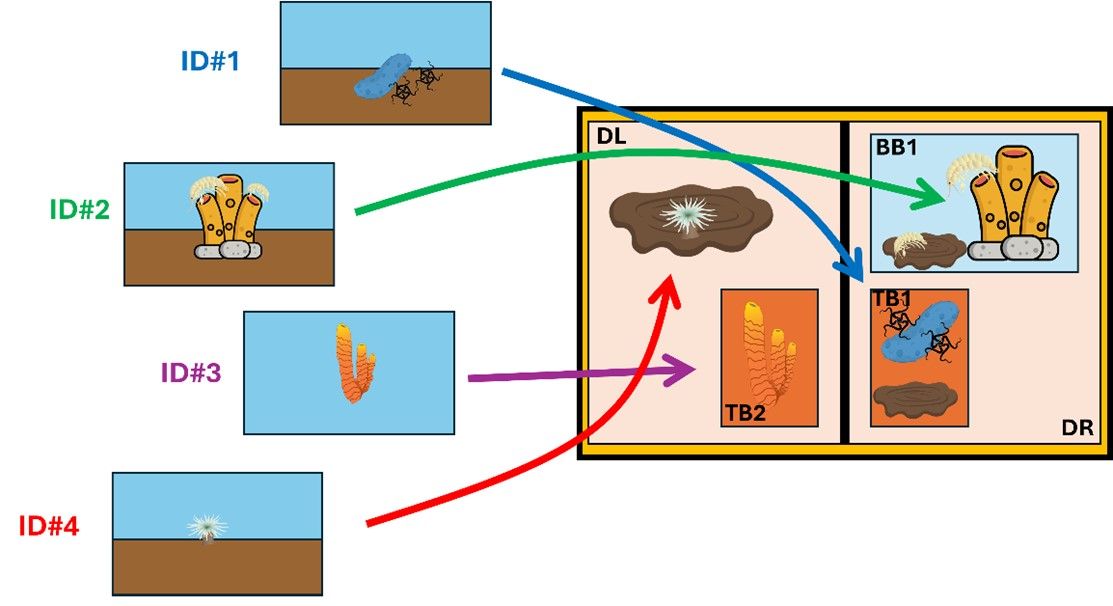
Once the ROV was on deck, processing containers (e.g., buckets and trays) were prelabelled with the Event ID and storage information for the different samples collected during the dive (Figure 8). The samples were retrieved from the storage compartments (namely, toolboxes, biobox in the left and right drawers, and suction sampler chambers) then deposited in the buckets with cool saltwater. Given that the two drawers from the ROV (left and right) were connected, it was impossible to attribute from which sample event the contents in them came from. Therefore, after each dive, a new sample event was given to the whole content of the drawer (both left and right). In that case, the whole content of the drawers was flushed through a side drain on top of a 300 µm sieve.
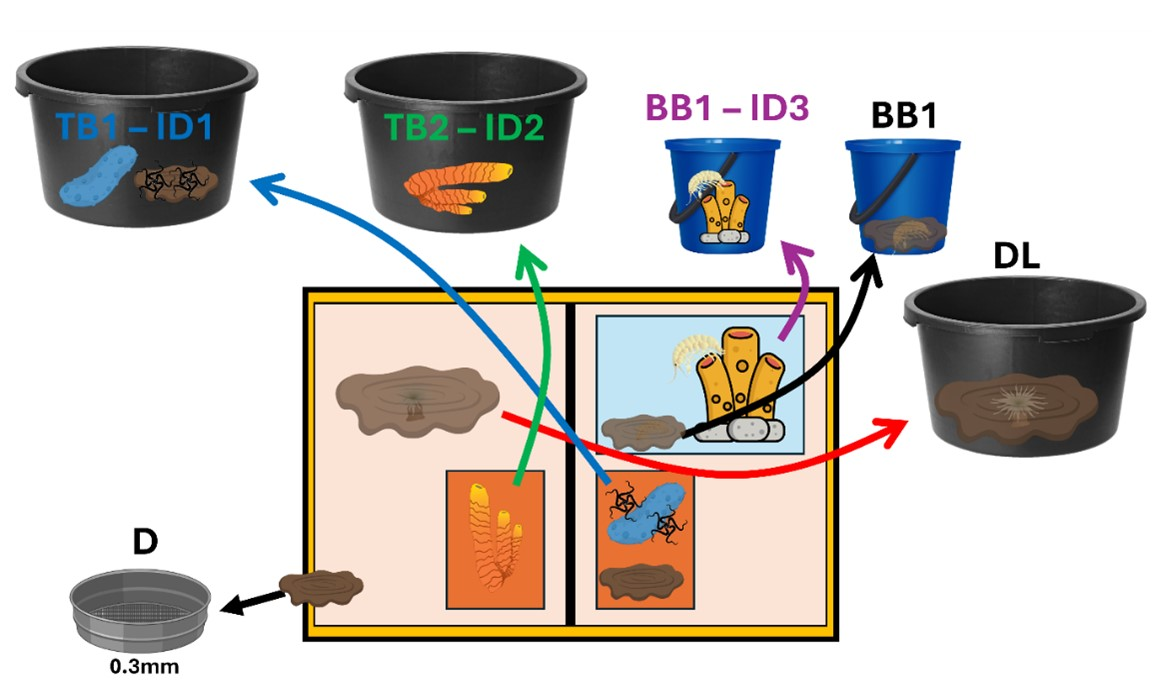
A similar situation occurred with the suction sampler since the chambers were not completely sealed, and some mixing could occur between them. Therefore, if multiple samples were stored in several chambers but some were left unused, the leftover content from the unused chambers was collected and sieved over a 300 µm sieve, and a new sample event number was assigned to the combined content from all unused chambers.
Once in the lab, the biological samples were sieved over a 300 µm sieve. For each sample, the targeted specimens seen on video were located and picked, photographed with a scale, and preserved in separate jars/vials with ethanol 96%. Other megafauna in the sample that was easily visible on video, although not directly targeted, were also picked, photographed, and preserved in separate jars/vials in ethanol 96%. All of the other associated fauna and sediment were bulk fixed with ethanol 96% for further sorting in the lab on land. Majority of the ROV sampling was qualitative (given that the area or volume of sample are unknown).
The only quantitative samples for biology taken by the ROV were the ones sampled with the blade corers. Upon retrieval, the blade corers were carefully placed and secured in tubs. Before opening, a picture of the side showing the sediment profile, was taken with a ruler for scale. After that, the plexiglass side of the core was unscrewed and the whole sediment content was poured into the tub. The sediment sample was then sieved through a sequential sieving of 1 mm, 500 µm and 300 µm, until all material was fractioned into the three meshes (see Section 3.4.6. Box Corer (0.25 m2) and figure 12 ). The content of each sieve was then fixed in ethanol 96% and labelled accordingly.
For all biological samples collected with the ROV, ethanol was changed after ca. 12 hours. Samples were kept cold (2 °C) and in the dark in a cooler room.
3.2.3 - ROV Chemical Sampling
Six push cores were taken for chemistry at one full station, R3753, with the purpose of comparing the results to those from traditional sampling with multi corer (see below). These push cores were collected in the same way as for geology (described above), but two of the cores were in stainless steel tubes. When onboard, the cores were retrieved and handled in the same way as described below for the multi corer, see Section 3.4.8. Multi Corer.
An extra sample for chemistry was taken at one full station, R3753, using blade corers adjusted for this purpose, with aluminum plates rather than transparent plexiglass plates used for biology (Photo 5). This was done with the purpose of comparing this way of sampling to traditional sampling done by box corer (see Section 3.4.6. Box Corer (0.10 m2)) for analysing contaminants of emerging concern (CECs). Since the blade corers with aluminum plates are not transparent, the blade corers were sunk into sediments roughly to the half of blade corer height, close to the opening of the metal plate in its middle, to have the sediment surface easily retrievable upon opening the side of the blade corer. Because the surface area of blade corers is smaller than what is necessary for obtaining three high quality samples, it was necessary to deploy two blade corers for this sampling. Due to risk of contamination, the blade corers were taken aside when onboard and only opened by the chemist after other personnel was away. The samples were then retrieved and handled in accordance with the traditional MAREANO procedure available at https://www.mareano.no/kart-og-data/kjemidata, see short description in Section 3.4.6 Box Corer (0.10 m2).

3.3 - Seabed Field Observer (SFO)
For annotating the dives live, an updated version of Seabed Field Observer (SFO) was used rather than using Campod Logger. Like the former SFO, the new SFO allowed annotators to log fauna, seabed features, litter, and operational comments as they occurred, as well as logging of the seabed at 10 second intervals. However, with the new SFO, habitat types for biology could now be logged at 10 second intervals as well. In addition to the standard Operational Commander (or the person who starts and stops a session and logs the operational comments (e.g., start/stop record, start transect, etc.)), two additional roles were added to the updated software, which were Seabed Annotator and Habitat Annotator who controlled the seabed and habitat interval logging, respectively.
For identifying the taxa observed during the ROV dives, we used the “Norwegian Deep Sea Image Catalogue” developed by Meyer, Zhulay, and Fredriksen in January 2025, which can be freely accessed here. The “Norwegian Deep Sea Image Catalogue” is a living catalogue that will continually be updated and was developed to form a standardization in the naming conventions of morphotaxa observed in visual data (e.g., imagery and videos) collected on and around AMOR.
3.4 - Full stations
Like in standard MAREANO cruises on the Norwegian Shelf, two full stations (per 1000 km2) were selected in each box for more intensive physical sample collection of benthic biological, geological, and chemical samples in addition to the video line. Modifications of the full station design were required to adjust to the conditions and time requirements for working in the deep sea (Figures 9 and 10), as proposed in the Deep-Sea Strategy (Ross et al., 2025).
3.4.1 - Standard MAREANO Full Station Design:
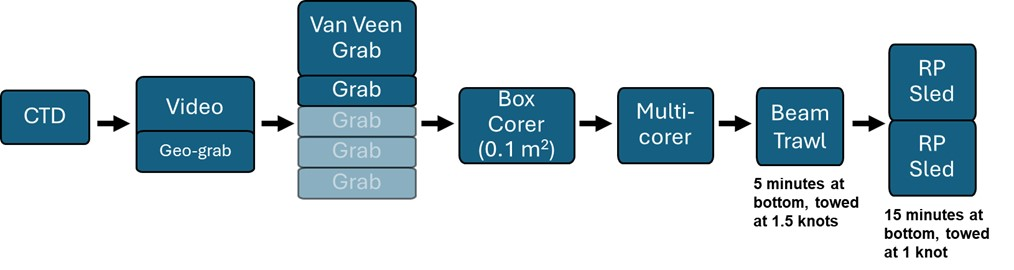
3.4.2 - Deep MAREANO Full Station Design:
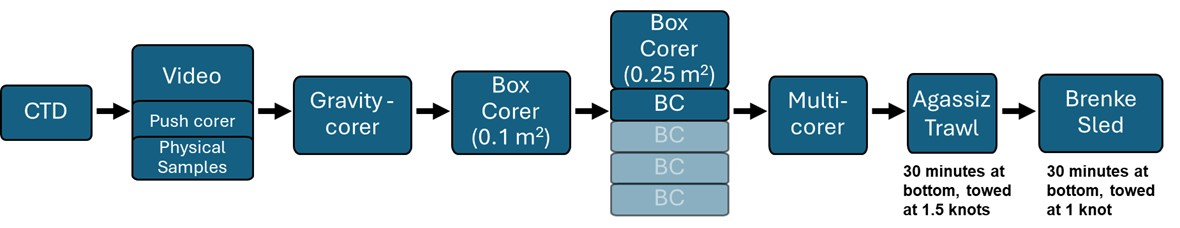
3.4.3 - Sub bottom profile and Multibeam
As for standard MAREANO cruises, sub bottom profile (SBP) and multibeam was collected when in transit between stations. However, due to the sensitivity of some of the gear (e.g., gravity corer) and the time it takes to deploy and retrieve gear in the deep sea, a more systematic acquisition of SBP was necessary for assessing the suitability of stations for physical sampling at the full stations. Therefore, unlike traditional MAREANO cruises, it was not possible to completely confirm the location of full stations until after the SBP (and video line) were performed to ensure the conditions suited the needs of the drop gear (hence the need for identifying potential full stations during the planning phase, Figures 2-4).
3.4.4 - CTD
Due to the complexity of the oceanographic setting on AMOR and the limited amount of raw oceanographic data points in the area, for this cruise CTD deployment was not only limited to the full stations as is the case for majority of the standard MAREANO cruises previously. However, the collection of bottom water from the CTDs were retained to full stations and regions with suspected increased or unique biodiversity such as hydrothermal vents and seamounts. We increased the number of CTD casts to gather a more representative coverage of the oceanographic conditions in each box to help with interpretation of the water masses in the region.
3.4.5 - Gravity Corer
Gravity cores were collected at the full stations with the aim of establishing sedimentation rates and genesis of the area (Photo 6). The gravity corer was borrowed from The Arctic University of Norway (UiT) due to shipping problems which caused parts of NGU’s gravity corer to not arrive in time for the cruise. A casing allowing core lengths of up to 6 m was used for both samplings. NORMAR ROV Ægir6000 filmed the sediment collection for both occasions but only recorded the latter. The cores were cut into 1 m sections and stored cold before shipment to NGU for further analysis.

3.4.6 - Box corer (0.10 m2)
In accordance with the standard MAREANO sampling strategy (see the detailed sampling procedure given at https://www.mareano.no/kart-og-data/kjemidata), a separate box corer (surface area 0.10 m2) was taken for sampling sediments to analyze CECs at one full station per area. The results of this sampling are to be compared to blade corer sampling, see Section 3.2.3 ROV Chemical Sampling. Precautions are necessary when taking this sample to avoid contamination. For this reason, the sampling location (on this cruise, R3753) was chosen in advance for the chemist to be able to prepare for the sampling. Other personnel were asked to keep aside when the box corer was opened. The box corer was taken to the side and opened when no one except the chemist was present. The field blank sample was opened at the same time as the box corer. The surface water was removed and a photo of the surface was taken. Three surface sediment samples (0-2 cm) were taken into glass jars which were sealed, marked and kept frozen until delivery to IMR laboratory together with the field blank sample.
3.4.7 - Box Corer (0.25 m2)
Due to the high risk of failure of operating Van Veen grabs at such great depths and to ensure comparability to international standards for sampling deep-sea infaunal biodiversity, a 0.25 m2 USNEL box corer was used to survey infauna in place of the Van Veen grab used in Standard MAREANO surveys. For this cruise, a USNEL box corer from Akvaplan-Niva was employed. At each box corer deployment, the ROV Ægir6000 stayed at the bottom to film live each of the landings and to ensure that the box corer was released properly.
Due to the lower macrofaunal diversity and species patchiness in the deep sea, box corer replicates were increased to 5 replicates at half of the full stations (the deepest full station per box) to precisely capture the macrofaunal diversity and species composition. The other half of the full stations had 2 box corer replicates, which corresponds with the usual number of Van Veen Grab replicates in MAREANO shelf surveys.
Given the foreseeable number of species unknown to science or poorly described that are expected in the deep sea, an end-to-end approach was applied (Figure 11). The box corer was split into two halves with the help of divider plates. After processing, one half of the box corer was fixed in ethanol 96%, and the other half was fixed in a 4% formaldehyde solution buffered with borax. This allows organisms fixed in ethanol to be genetically barcoded and then linked to morphologically described taxa in the formalin half.
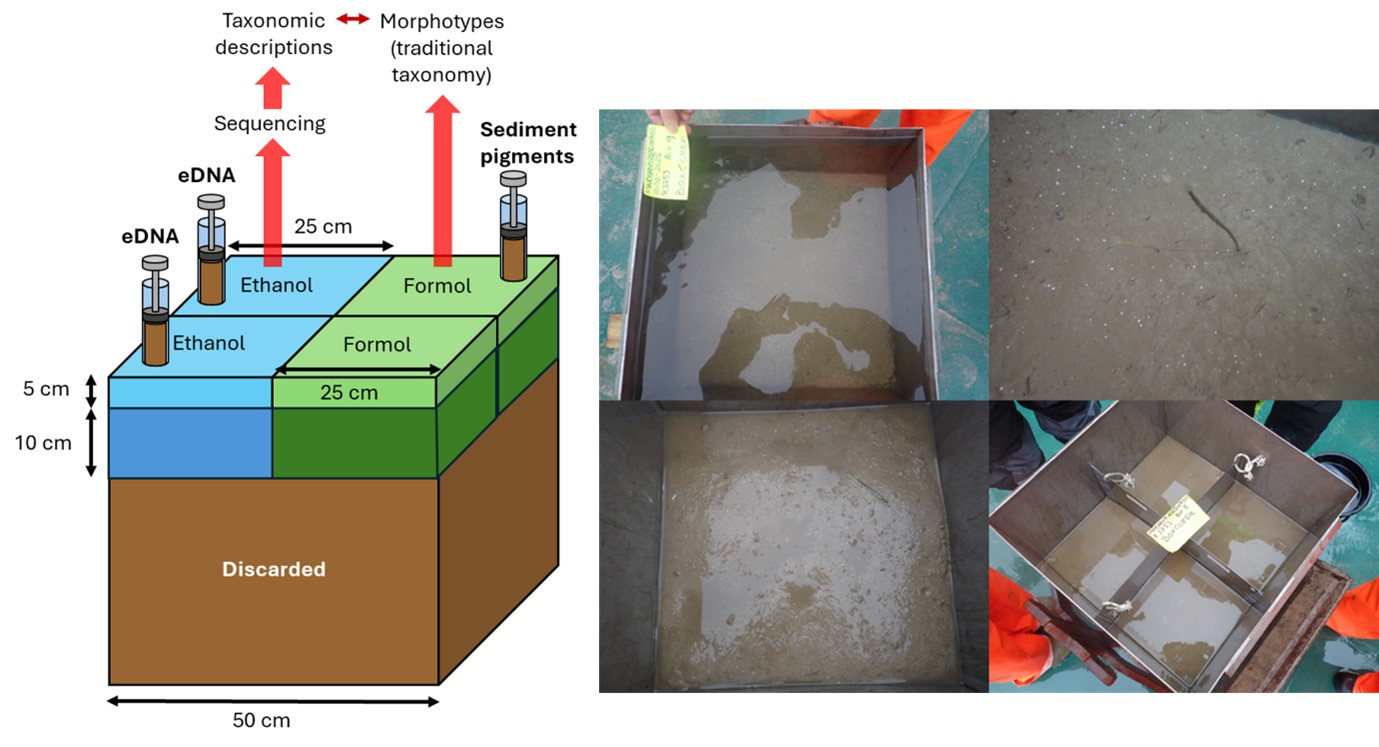
The two halves were further split vertically into two layers: the surface layer between 0-5 cm and the deeper layer between 5-15 cm. The overlaying water was carefully siphoned out with a hose over a 300 µm sieve to capture any hyperbenthic organisms. After the overlaying water was removed and the sediment surface was documented photographically, the splitters were inserted. At this point, two eDNA samples were taken from the ethanol preserved half and one sample replicate for sediment pigments was taken with a cut-off syringe from 0-2 cm sediment depth on the formalin half. After that, the two vertical layers of 0-5 cm were scooped out gently into buckets, keeping the two halves separate (ethanol vs formalin).
In the lab, the 0-5 cm layer was sieved over a sequential sieving procedure, where the sediment samples were passed through a 1 mm sieve, followed by a 500 µm sieve and lastly, a 300 µm sieve (Figure 12). The remaining sediment passing through the 300 µm sieve was discarded. This procedure was done for both halves of the box core, and the sieve contents were ultimately preserved and labelled in respective jars with either ethanol or formalin.
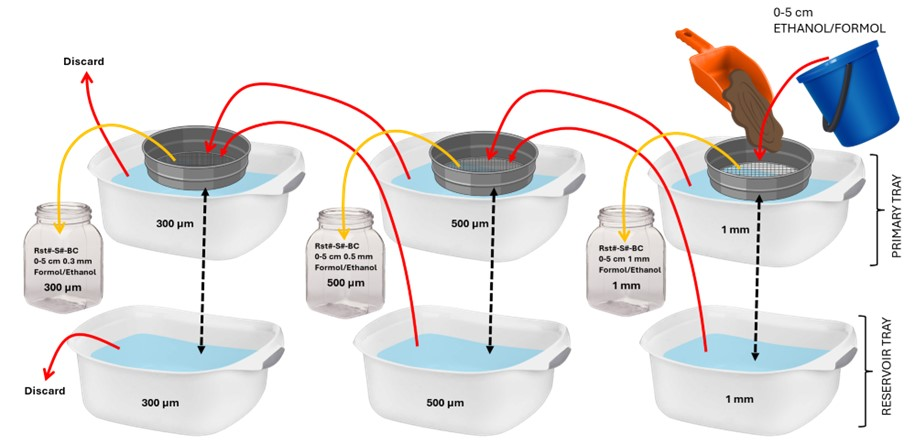
The 5-15 cm layer was then scooped out into tubs and poured into a hand-made elutriation device, which was built for this cruise partially following the device descriptions in Santos et al. 1996 (Figure 13). Each half of the box corer (ethanol/formalin) for the 5-15 cm layer was poured into a tank with a saltwater inflow at the bottom. The water uptake connection to the tank had a 200 µm mesh to avoid losing any part of the sample. Once the device was turned on, the elutriated fraction left the tank through an outflow hole in the upper part of the tank connected to a hose that led to a partially submerged 300 µm sieve. The sample was left elutriating for about 1 to 1.5 hours, gently stirring the surface every now and then. The elutriated fraction of the 300 µm sieve was then bulk fixed in ethanol or in formalin respectively, for the different halves of the box core. Finally, the remaining fraction in the tanks (what we refer to as “heavy fraction”) was sieved over a 300 µm sieve and both halves were combined into a large bucket and fixed with formalin 4% solution and borax.
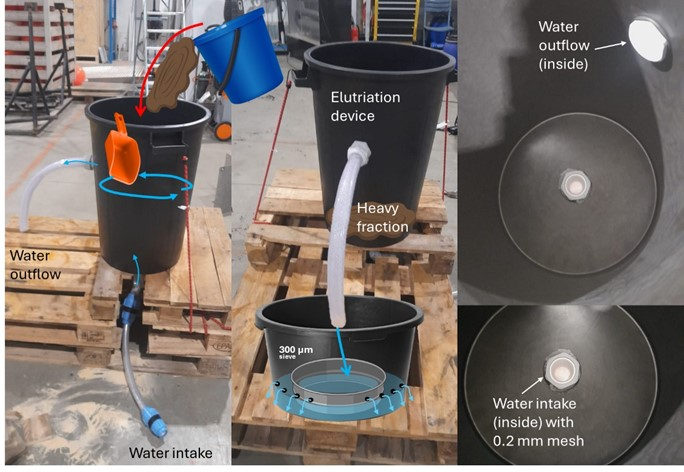
All ethanol fixed samples from the box corer were kept in a cold room (2 oC) and kept in the dark.
3.4.8 - Multi corer
Like in standard MAREANO cruises (see the detailed sampling procedure given at https://www.mareano.no/kart-og-data/kjemidata), multi corers were conducted at the full stations. Six cores are obtained with each multi corer deployment, four in plastic tubes and two in stainless steel tubes (Photo 7). Once on board, we check if enough cores are approved (3 in plastic tubes, 1 in the steel tubes), measure their height and decide the owner (A-E). Photos are taken of the cores with a scale, label and core number on the frame, including pictures of both sides of the multi corer. We also take photos of the surface of core A and B before slicing them and describe core A in detail (on paper + Survey 123). Core A is the longest plastic core, and the slices are sent for inorganic contaminants, grain size and other sediment characteristics measurements at NGU. Core B is a plastic tube core for measuring organic contaminants at IMR. The two shortest plastic tube cores and two steel tube cores are sealed for later measurements such as XRI and microplastics at NGU and IMR. Due to expected low sedimentation rates, we subsample the sliced cores, slicing them into 0.5 cm thick slices in the top 10 cm of the cores and 1 cm slices below (normal MAREANO standard is 1 cm). Sliced samples were stored frozen (-18°C) and cores stored at room temperature.

3.4.9 - Agassiz Trawl
Due to the depths and risk of failure in case the beam trawl landed incorrectly and to ensure comparability to international standards for sampling epifaunal biodiversity, the Agassiz trawl was used to capture the physical epibenthic samples in the full station. However, due to a difference in mesh sizes between the two gears where the inner mesh of the Agassiz trawl was approximately 1 cm and the inner mesh of the Beam Trawl was approximately 5 mm, the Beam Trawl was also used in both stations to determine if the catch was vastly different and if the gear is not suitable for the conditions.
The wire length used when deploying the gear was dependent on the depth, where the length was decreased with increasing depth to reduce the amount of slack on the wire as per recommendation from technicians, deck crew and captain (e.g., those experienced with trawling). The ideal wire length for trawling at great depths is between 1.3 to 1.7x the depth of the station. The trawl was set out at 1.5 knots until the depth stopped decreasing, then the ship stood still for 15 minutes to allow the trawl to reach the seafloor. Then the trawl was towed at 1.5 knots for 30 minutes.
Following standard MAREANO procedures, samples were photographed and generally sieved over a 5 mm sieving table. However, the deep-sea fauna is characterized by thinner, less heavily mineralized calcium carbonate structures (e.g., shells, skeletons) and are, therefore, generally more fragile during sampling and processing than their shallow-water counterparts. Thus, in some cases, it was decided to sieve the catch over a 2 mm sieve to avoid destroying delicate fauna on the grids of the sieving table. In MAREANO standards, a 1 mm sieve is usually placed under the sieving table to capture smaller fauna. However, given that the body size of macro- and megafauna decreases exponentially towards the deep sea, a 0.5 mm sieve was used instead. All content from the catch was fixed in ethanol 96%. After ca. 12 hours, the ethanol of the samples was exchanged. The 0.5 mm fraction was sent to the University Museum in Bergen (UMB). Samples of fish and cephalopods were frozen in -20°C. For abundant fish species that could be identified to species level on board, individuals were counted, weighed and then discarded, as no further taxonomic inspection was required.
3.4.10 - Brenke Sled
While we attempted to acquire a Brenke Sled for this cruise for collecting hyperbenthos and small epifauna near the sediment surface (Brenke, 2005), we were unable to and had to use MAREANO’s RP Sled instead. However, we used the modifications that were suggested for the Brenke Sled for deep-sea sampling on the RP Sled.
Only one sled would be deployed per full station unless the first sled failed. The RP sled was slowly set out to the wire length before the ship stopped for 15 minutes to allow the sled to reach the seafloor. The sled was towed at 1 knot for 30 minutes.
We followed the processing procedures used in standard MAREANO cruises.
4 - Activity Timetable
| Day # | Date | Time | P # | R # | Activity # | Activity |
| 1 – Tuesday | 23.09.2025 | 08:00 | Mobilization | |||
| 2 – Wednesday | 24.09.2025 | 08:00 | Left Isfjorden | |||
| 24.09.2025 | Transit | |||||
| 24.09.2025 | 13:25 | ROV Test Dive | ||||
| 3 – Thursday | 25.09.2025 | Transit | ||||
| 4 – Friday | 26.09.2025 | 09:00 | Multibeam and SBP | |||
| 26.09.2025 | 10:00 | P14 | 3740 | 454 | CTD | |
| 26.09.2025 | 12:20 | P14 | 3740 | 3831/1024 | ROV | |
| 26.09.2025 | 18:30 | Standby due to weather | ||||
| 5 – Saturday | 27.09.2025 | Standby due to weather | ||||
| 6 – Sunday | 28.09.2025 | Standby due to weather | ||||
| 28.09.2025 | 04:00 | 3741 | 37 | Multibeam | ||
| 28.09.2025 | 04:00 | 3741 | 38 | Sub Bottom Profiler | ||
| 28.09.2025 | 6:20 | P70 | 3741 | 3832/1025 | ROV | |
| 28.09.2025 | 12:15 | P15 | 3742 | 455 | CTD | |
| 28.09.2025 | 13:30 | P15 | 3742 | 3833/1026 | ROV | |
| 28.09.2025 | 20:10 | P13 | 3743 | 3834/1026 | ROV | |
| 7 – Monday | 29.09.2025 | 00:10 | 3744 | 39 | Multibeam | |
| 29.09.2025 | 00:10 | 3744 | 40 | Sub Bottom Profiler | ||
| 29.09.2025 | 02:55 | P81 | 3744 | 456 | CTD | |
| 29.09.2025 | 04:30 | P81 | 3744 | 3835/1027 | ROV | |
| 29.09.2025 | 09:00 | P81b | 3744 | 3836/1027 | ROV | |
| 29.09.2025 | 15:00 | P16 | 3745 | 3837/1028 | ROV | |
| 29.09.2025 | 21:00 | P17 | 3746 | 3838/1028 | ROV | |
| 8 – Tuesday | 30.09.2025 | 01:10 | 3747 | 41 | Multibeam | |
| 30.09.2025 | 01:10 | 3747 | 42 | Sub Bottom Profiler | ||
| 30.09.2025 | 02:10 | P88 | 3747 | 457 | CTD | |
| 30.09.2025 | 03:35 | P88 | 3747 | 3839/1029 | ROV | |
| 30.09.2025 | 08:30 | 3748 | 43 | Multibeam | ||
| 30.09.2025 | 08:30 | 3748 | 44 | Sub Bottom Profiler | ||
| 30.09.2025 | 10:35 | P87 | 3748 | 458 | CTD | |
| 30.09.2025 | 13:00 | P87 | 3748 | 3840/1030 | ROV | |
| 30.09.2025 | 22:00 | P1 | 3749 | 3841/1031 | ROV | |
| 9 – Wednesday | 01.10.2025 | 02:10 | P7 | 3750 | 3842/1031 | ROV |
| 01.10.2025 | 09:10 | P86 | 3751 | 3843/1032 | ROV | |
| 01.10.2025 | 15:00 | 3752 | 45 | Multibeam | ||
| 01.10.2025 | 15:00 | 3752 | 46 | Sub Bottom Profiler | ||
| 01.10.2025 | 16:50 | P3 | 3752 | 459 | CTD | |
| 01.10.2025 | 19:00 | Standby due to weather | ||||
| 10 – Thursday | 02.10.2025 | Standby due to weather | ||||
| 11 – Friday | 03.10.2025 | Standby due to weather | ||||
| 03.10.2025 | 07:05 | P8 | 3753 | 460 | CTD | |
| 03.10.2025 | 08:55 | P8 | 3753 | 3844/1033 | ROV | |
| 03.10.2025 | 17:05 | P8 | 3753 | 1 | Box Corer (0.1m2) | |
| 03.10.2025 | 20:25 | P8 | 3753 | 2 | Box Corer (0.25m2) – Failed | |
| 03.10.2025 | 23:00 | P8 | 3753 | 3/4 | Box Corer (0.25m2) – Failed | |
| 12 – Saturday | 04.10.2025 | 23:35 | P8 | 3753 | 3/4 | Box Corer (0.25m2) – Not new. |
| 04.10.2025 | 03:50 | P8 | 3753 | 1 | Agassiz Trawl | |
| 04.10.2025 | 07:05 | P8 | 3753 | 1 | RP Sled | |
| 04.10.2025 | 09:00 | P68 | 3754 | 3845/1034 | ROV | |
| 04.10.2025 | 16:45 | P3 | 3752 | 3846/1035 | ROV | |
| 13 – Sunday | 05.10.2025 | 00:05 | P69 | 3755 | 3847/1036 | ROV |
| 05.10.2025 | 07:10 | 3756 | 47 | Multibeam | ||
| 05.10.2025 | 07:10 | 3756 | 48 | Sub Bottom Profiler | ||
| 05.10.2025 | 08:00 | P82a | 3756 | 461 | CTD | |
| 05.10.2025 | 11:50 | P82a | 3756 | 3848/1037 | ROV | |
| 05.10.2025 | 18:00 | P82b | 3757 | 3849/1037 | ROV | |
| 14 – Monday | 06.10.2025 | 01:30 | P82c | 3758 | 3850/1037 | ROV |
| 06.10.2025 | 08:45 | P8 | 3753 | 1 | Gravity corer | |
| 06.10.2025 | 11:30 | P8 | 3753 | 5 | Box Corer (0.25m2) | |
| 06.10.2025 | 13:55 | P8 | 3753 | 6 | Box Corer (0.25m2) | |
| 06.10.2025 | 16:20 | P8 | 3753 | 1 | Multicorer | |
| 06.10.2025 | 21:25 | P8 | 3753 | 2 | RP Sled - Failed | |
| 15 – Tuesday | 07.10.2025 | 01:30 | P8 | 3753 | 2 | Beam Trawl |
| 07.10.2025 | 05:50 | 3759 | 49 | Multibeam | ||
| 07.10.2025 | 05:50 | 3759 | 50 | Sub Bottom Profiler | ||
| 07.10.2025 | 06:05 | Standby due to weather | ||||
| 16 – Wednesday | 08.10.2025 | Standby due to weather | ||||
| 08.10.2025 | 19:55 | P10 | 3759 | 462 | CTD | |
| 08.10.2025 | 22:05 | P10 | 3759 | 3851/1039 | ROV | |
| 17 – Thursday | 09.10.2025 | 05:00 | 3760 | 51 | Multibeam | |
| 09.10.2025 | 05:00 | 3760 | 52 | Sub Bottom Profiler | ||
| 09.10.2025 | 06:15 | P9 | 3760 | 462 | CTD | |
| 09.10.2025 | 08:00 | P9 | 3760 | 3852/1040 | ROV | |
| 09.10.2025 | 15:20 | P84 | 3761 | 3853/1041 | ROV | |
| 09.10.2025 | 21:15 | P85 | 3762 | 3854/1042 | ROV | |
| 18 – Friday | 10.10.2025 | 04:30 | P8 | 3753 | 7 | Box Corer (0.25m2) |
| 10.10.2025 | 07:30 | P8 | 3753 | 8 | Box Corer (0.25m2) | |
| 10.10.2025 | 09:25 | P8 | 3753 | 9 | Box Corer (0.25m2) | |
| 10.10.2025 | 11:50 | 3763 | 53 | Multibeam | ||
| 10.10.2025 | 11:50 | 3763 | 54 | Sub Bottom Profiler | ||
| 10.10.2025 | 14:00 | P66 | 3763 | 464 | CTD | |
| 10.10.2025 | 16:00 | P66 | 3763 | 3855/1044 | ROV | |
| 10.10.2025 | 21:00 | P66 | 3763 | 2 | Gravity corer | |
| 19 – Saturday | 11.10.2025 | 00:00 | P66 | 3763 | 10 | Box Corer (0.25m2) |
| 11.10.2025 | 02:00 | P66 | 3763 | 11 | Box Corer (0.25m2) | |
| 11.10.2025 | 04:45 | P66 | 3763 | 2 | Multicorer | |
| 11.10.2025 | 07:50 | P66 | 3763 | 3 | RP Sled - Failed | |
| 11.10.2025 | 12:40 | P66 | 3763 | 4 | RP Sled | |
| 11.10.2025 | 17:45 | P66 | 3763 | 3 | Beam Trawl | |
| 11.10.2025 | 22:50 | P66 | 3763 | 4 | Agassiz Trawl | |
| 20 – Sunday | 12.10.2025 | 01:25 | P4 | 3764 | 3856/1046 | ROV |
| 12.10.2025 | 07:05 | P67 | 3765 | 3857/1047 | ROV | |
| 12.10.2025 | 13:20 | P6 | 3766 | 3858/1048 | ROV | |
| 12.10.2025 | 19:25 | P11 | 3767 | 3859/1048 | ROV - Aborted | |
| 12.10.2025 | 21:55 | 3768 | 55 | Sub Bottom Profiler | ||
| 12.10.2025 | 22:00 | 3768 | 54 | Multibeam | ||
| 21 – Monday | 13.10.2025 | 02:20 | P2 | 3768 | 3860/1049 | ROV |
| 13.10.2025 | 08:00 | 3769 | 55 | Multibeam | ||
| 13.10.2025 | 08:00 | 3769 | 56 | Sub Bottom Profiler | ||
| 13.10.2025 | 08:20 | P2 | 3769 | 3861/1050 | ROV | |
| 13.10.2025 | 14:40 | P11 | 3767 | 3862/1051 | ROV | |
| 13.10.2025 | 19:50 | 3769 | 56 | Multibeam | ||
| 13.10.2025 | 20:30 | 3769 | 57 | Sub Bottom Profiler | ||
| 13.10.2025 | 21:00 | Standby due to weather | ||||
| 22 – Tuesday | 14.10.2025 | Standby due to weather | ||||
| 14.10.2025 | 11:30 | P83 | 3770 | 3863/1052 | ROV | |
| 14.10.2025 | 17:10 | 3771 | 58 | Multibeam | ||
| 14.10.2025 | 17:10 | 3771 | 59 | Sub Bottom Profiler | ||
| 14.10.2025 | 18:25 | P12 | 3771 | 3864/1053 | ROV | |
| 14.10.2025 | 23:00 | 3772 | 59 | Multibeam | ||
| 14.10.2025 | 23:00 | 3772 | 60 | Sub Bottom Profiler | ||
| 23 – Wednesday | 15.10.2025 | 05:50 | P90 | 3772 | 465 | CTD |
| 15.10.2025 | 6:40 | P90a | 3772 | 60 | Multibeam | |
| 15.10.2025 | 6:40 | P90a | 3772 | 61 | Sub Bottom Profiler | |
| 15.10.2025 | 07:00 | P90a | 3772 | 3865/1054 | ROV | |
| 15.10.2025 | 14:30 | P90b | 3773 | 3866/1055 | ROV | |
| 15.10.2025 | 21:30 | P90c | 3774 | 3867/1055 | ROV | |
| 24 – Thursday | 16.10.2025 | 02:50 | P20 | 3775 | 3868/1056 | ROV |
| 16.10.2025 | 08:50 | 3776 | 62 | Multibeam | ||
| 16.10.2025 | 08:50 | 3776 | 63 | Sub Bottom Profiler | ||
| 16.10.2025 | 11:20 | P29 | 3776 | 466 | CTD | |
| 16.10.2025 | 12:30 | P29 | 3776 | 3869/1057 | ROV | |
| 16.10.2025 | 16:05 | P30 | 3777 | 3870/1057 | ROV | |
| 16.10.2025 | 21:25 | P71 | 3778 | 3871/1058 | ROV | |
| 25 – Friday | 17.10.2025 | 04:00 | P71b | 3779 | 3872/1059 | ROV |
| 17.10.2025 | 12:30 | Left AMOR | ||||
| 17.10.2025 | Transit | |||||
| 26 – Saturday | 18.10.2025 | Transit | ||||
| 27 – Sunday | 19.10.2025 | Transit | ||||
| 19.10.2025 | 13:00 | Arrive in Longyearbyen | ||||
| 28 – Monday | 20.10.2025 | 08:00 | Demobilization |
5 - Time Spent Overview
We completed 41 video lines over 42 ROV dives making up 164x 200 m long transects and 2 full stations in 16 days (Table 4). We have lost 5 days due to variables outside of our control, such as weather or gear failure (explained in detail below in Section 8. Limitations). The time lost does not include expected delays due to transit time or gear deployment. When conditions allowed, we averaged 3 to 4 dives per 24-hour period. On average, each ROV dive from ROV deployment to recovery took approximately 5.5 hours, and each 800 m video line took approximately 2.5 to 3.5 hours to complete depending on the sampling intensity.
| Activity | Total Time Spent (Hours) | Total Time Spent (Days) |
| Mobilization | 24 | 1 |
| Demobilization | 24 | 1 |
| Transit to/from Longyearbyen | 117.5 | 5 |
| Work | 384.5 | 16 |
| Standby due to weather | 120 | 5 |
6 - Cruise Summary
By the end of the cruise 2025007011, all of B06 and part of B07 were completed (Figure 14). We completed 33 stations with 2 full stations in B06 and 8 stations in B07 (no full stations).
6.1 - Video Lines
There were 77 push corers taken, approximately 19 rock samples collected, 10 chemical sampling events, and 105 biological sampling events over the course of the 41 video lines.
During the cruise, we adjusted the location and lengths of some of the video lines due to incorrect initial placement or not taking the steep terrain into consideration in the planning phase. In NH3-B06, it was realized that the location of Ægir Spring (P82) was not correct and thus was readjusted once we were closer to the station. We also made the decision to add two more video lines at P82 to cover the geo- and biodiversity of the venting and background area since MAREANO has relatively limited experience mapping around hydrothermal vent fields. In NH3-B07, we decided to add 2 more targeted video lines on P90 and adjust its initial location and 1 more video line on P71 to cover the depth gradients on the seamounts since studies have shown biotope zonation patterns on seamounts on AMOR that would have otherwise been missed.
6.1.1 - Biology
While SFO does not provide exact numbers or a thorough analysis of the visual data, general trends of relative abundances and richness at the stations can be observed using the software. It must also be stated that general biases cannot be overlooked when examining the data, such as: 1) the loggers’ experiences improved throughout the cruise as they became more familiar with the taxa and habitats; 2) the number of people available for logging where logging would be better if a "caller" was available to call out taxa observed on the screen; and 3) loggers’ distance to the screen. Therefore, the results presented in this cruise report simply visualize the trends that were observed and should not be taken as completed analysis of the visual data.
Based on the field reports (and preliminary data) generated with SFO, there were 166 morphotaxa and 511 029 individuals logged in SFO. Echinoderms had the most individuals logged across the dives, with 220 947 individuals, followed by Porifera (89 034 individuals), and Annelids (71 844 individuals) (Figure 15). Porifera were the most diverse group amongst the dives with 44 morphotaxa otus logged, followed by Cnidarians (25 otus), and Echinoderms (24 otus).
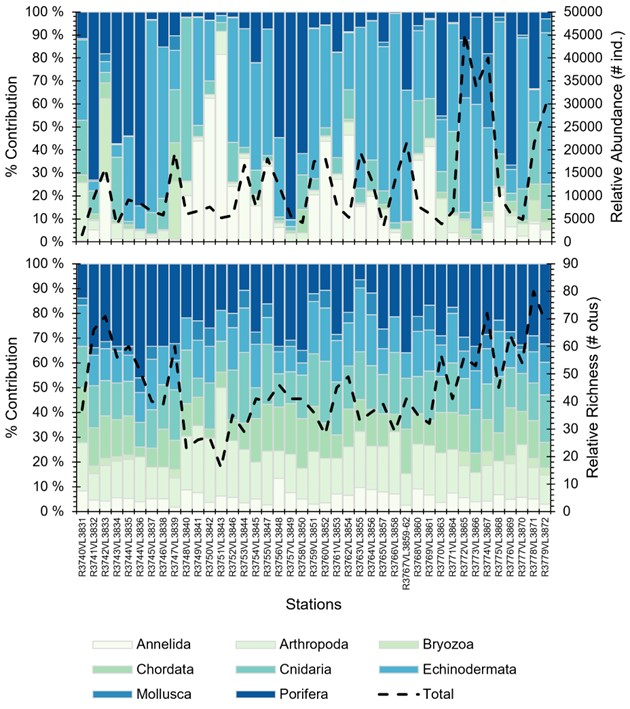
The station with the least and most individuals logged were R3740VL3831 (P14) and R3772VL3865 (P90a) with 1 539 and 45 283 individuals, respectively. The station with the least and most morphotaxa logged were R3751VL3843 (P86) and R3776VL3871 (P71) with 16 and 80 otus, respectively.
To examine the preliminary community trends from the SFO data, preliminary cluster analysis were conducted on the dataset. An Indicator Species Analysis was then performed on the clusters to identify which morphotaxa were most consistently present within the proposed clusters. More detailed annotation and analysis of the visual data is required to identify the biotopes present in the region and the clusters presented here is just a “first glimpse” into the main habitats that were observed during the cruise. Many of the morphotaxa observed were labeled with video names that require further examination from experts and physical samples to confirm the identifications. Therefore taxa names may change or differ in the future as identifications are confirmed.
| Cluster | Habitat | Depth Range (m) | Main Sessile Taxa | Main Mobile Taxa |
| 1 | Neohela field with anemones | 1640-2145 | Neohela sp., Actiniaria dark purple, Actiniaria epizoic, Bathycrinus carpenterii, Thenea sp., Antedonoidea, | Amphipoda, Bythocaris sp., Neobirsteiniamysis inermis, Prosobranchia, Hymenaster pellucidus, Mysidae, Lycodes frigidus, Pourtalesia jeffreysi, Lycodes sp, Polynoidea |
| 2 | Geodia sponge ground/wall | 1610-1980 | Geodia parva/Stelletta raphidiophora, Geodia hentscheli, Geodia sp., Amphidiscella monai, Aphrocallistidae, Lissodendoryx (Lissodendoryx) complicate, Asbestopluma furcata, Craniella sp., Cladorhizidae, Polymastiidae, Spinularia sp., Porifera fan, Bathycrinus carpenterii, Antedonoidea, Actiniaria, Actiniaria dark purple Sabellidea, Neohela sp. | Amphipoda, Bythocaris sp., Neobirsteiniamysis inermis, Prosobranchia, Hymenaster pellucidus, Mysidae, Lycodes frigidus, Lycodes sp, Amathillopsis spinigera, Ptychogastria polaris, Polynoidea, Tylaster willei |
| 3 | Glass sponge ground | 1080-1910 | Schaudinnia/Trichasterina/Scyphidium, Gersemia, Ciona intestinallis longissima, Molgulidae Actinostolidae, Hormathiidae, Actiniaria, Actiniaria epizoic, Bryozoa, Idmidronea, Antedonoidea, Sabellidae, Serpulidae, Geodia hentscheli, Geodia sp., Craniella sp., Asbestopluma furcata, Cladorhiza sp., Cladorhizidae, Polymastia thielei, Spinularia sp., Polymastiidae, Porifera off white irregular, Porifera fan | Gaidropsarus.argentatus, Amphipoda. Bythocaris sp., Neobirsteiniamysis inermis, Mysidae, Tylaster willei, Hymenaster pellucidus, Asteroidea, Prosobranchia, Ptychogastria polaris, Polynoidea |
| 4 | Hard bottom sponge ground with crinoids (unstalked) | 2000-2390 | Lissodendoryx (Lissodendoryx) complicata, Spinularia sp., Polymastiidae, Porifera small round, Amphidiscella monai, Aphrocollistidae, Porifera encrusting, Porifera fan, Geodia parva/Stelletta rhaphidiophora, Geodia hentscheli, Geodia sp., Antedonoidea, Bathycrinus carpenterii, Ascidiacea colonial encrusting, Gersemia, Actinostolidae, Actiniaria dark purple, Actiniaria epizoic, Neohela sp., | Amphipoda, Bythocaris sp., Neobirsteiniamysis inermis, Mysidae, Prosobranchia, Asteroidea, Hymenaster pellucidus, Lycodes frigidus, Lycodes sp. |
| 5 | Bathycrinus field with Sabellidae and anemones | 2375-3270 | Bathycrinus carpenterii, Sabellidae, c.f.Bathyphellia sp., Actiniaria dark purple, Actiniaria epizoic | Amphipoda, Bythocaris sp., Mysidae, Lycodes frigidus, Lycodes sp. |
| 6 | Bamboo coral reef | 935-995 | Keratoisidiidae, Idmidronea, Bryozoa, Hydrozoa bush, Gersemia sp., Actinostolidae, Actiniaria epizoic, Ciona intestinallis longissima, Ascidia obliqua, Molgulidae, Antedonoidea, Schaudinnia/Trichasterina/Scyphidium, Lissodendoryx (Lissodendoryx) complicata, Hemigellius sp., Cladorhiza sp., Asbestopluma furcata, Cladorhizidae,Porifera white epibiont, Porifera fan, Craniella sp., Hexadella dedritifera, Stylocordyla borealis, Polymastiidae, Limatula sp., Pectinidae, Serpulidae | Gaidropsarus.argentatus, Amblyraja hyperborea, Tylaster willei, Asteroidea, Ophiuroidea, Prosobranchia, Caridea, Neobirsteiniamysis inermis, Mysidae |
| 7 | Bathyphellia and Sabellidae tube field with glass sponges | 3225-3255 | c.f.Bathyphellia sp., Sabellidae, Asconema megaatrialia Porifera small, Actiniaria | Amphipoda, Bythocaris sp., Lycodes sp. |
| 8 | Sabellidae tube field with Bathycrinus | 2870-3340 | Sabellidae, Bathycrinus carpenterii, Thenea sp., c.f.Bathyphellia sp., | Pourtalesia jeffreysi, Eplidia sp., Amphipoda, Bythocaris sp., Mysidae, Pycnogonida, Lycodes frigidus |
| 9 | Kolga aggregation in Bathycrinus and/or Sabellidae tube field | 2270-2680 | Bathycrinus carpenterii, Sabellidae, c.f.Bathyphellia sp., Actiniaria dark purple, Actiniaria epizoic, Thenea sp., | Kolga hyalina, Pourtalesia jeffreysi, Amphipoda, Neobirsteiniamysis inermis, Pycnogonida, Hymenaster pellucidus, Mysidae, Lycodes frigidus, Lycodes sp., Prosobranchia |
| 10 | Bathycrinus and Sabellidae field with anemones and Kolga | 2400-2925 | Bathycrinus carpenterii, Sabellidae, c.f.Bathyphellia sp., Actiniaria dark purple, Polymastiidae, Thenea sp., | Amphipoda, Neobirsteiniamysis inermis, Hymenaster pellucidus, Mysidae, Pycnogonidae, Eplidia sp., Lycodes frigidus, Pourtalesia jeffreysi, Lycodes sp., Prosobranchia, |
| 11 | Hard bottom sponge aggregation (Polymastiidae, encrusting, branching, and fan sponges) | 2270-2560 | Polymastiidae, Lissodendoryx (Lissodendoryx) complicata, Amphidiscella monai, Asconema megaatrialia, Hymedesmiidae, Porifera fan, Bathycrinus carpenterii, Antedonoidea, Actiniaria, Actiniaria dark purple, Ascidiacea colonial encrusting | Amphipoda, Bythocaris sp., Neobirsteiniamysis inermis, Mysidae, Lycodes frigidus, Lycodes sp. |
| 12 | Glass sponge ground with Ophiuroid bed | 915-1390 | Schaudinnia/Trichasterina/Scyphidium, Geodia sp., Polymastia thielei, Polymastiidae, Asbestopluma furcata, Cladorhiza sp., Cladorhizidae, Hexadella detritifera, Porifera encrusting, Ciona intestinallis longissima, Molgulidae, Antedonoidea, Gersemia sp., Actinostolidae, Hormathiidae, Actiniaria, Hydrozoa bryozoa soft bush, Idmidronea, Serpulidae, Sabellidae, Limatula, Pectinidae, Scalpellidae | Ophiuroidea, Gaidropsarus.argentatus, Amphipoda, Caridea, Neobirsteiniamysis inermis, Tylaster willei, Hymenaster pellucidus, Asteroidea, Ptychogastria polaris, Prosobranchia |
| 13 | Neohela aggregation with Geodia patches | 1335-1495 | Neohela sp., Geodia parva/Stelletta rhaphidiophora, Porifera encrusting, Polymastia thielei, Spinularia sp., Polymastiidae, Porifera off white irregular, Bathycrinus carpenterii, Actinostolidae, Actiniaria dark purple, Actiniaria, Serpulidae | Ophiuroidea, Amathillopsis spinigera, Amphipoda, Bythocaris sp., Caridea, Neobirsteiniamysis inermis, Mysidae, Tylaster willei, Hymenaster pellucidus, Asteroidea, Lycodes frigidus, Lycodes sp., Ptychogastria polaris, Polynoidea, Prosobranchia, |
Overall, 13 Clusters were identified. The initial main splitting in the cluster analysis is consistent with substrate type, where clusters 5, 8, 9, and 10 corresponded with purely deep soft bottom communities, and while the remaining clusters generally corresponded with comparatively shallower and/or hard(er) bottom communities. Many of the identified habitats are consistent with what was described by Meyer et al., 2023.
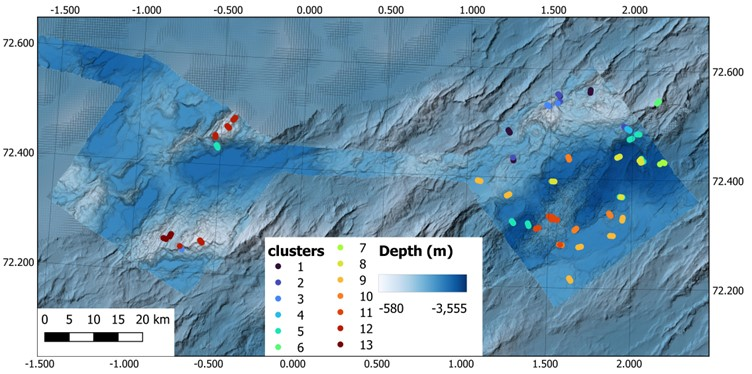
Cluster 1 and 13 is consistent with the Neohela fields with anemones and agglutinated foraminifera observed between 1640 and 2145 m depth (Figure 17A & O). Cluster 2 is categorized by Geodia sponge grounds/walls found at 1610-1980 m depth and is dominated by Geodia and Stelletta sponges on either soft bottom or hard susbtrate (Figure 17B & C). Cluster 3 and 12 are glass sponge grounds found between 915 and 1910 m depth primarily formed by the glass sponges Schaudinnia rosea, Trichasterina borealis, Scyphidium septentrionale (Figure 17D & M). Cluster 4 and 11 were dominated by hard bottom sponges (polymastids, encrusting, branching, and fan sponges) and Antedonoidea crinoids from 2000-2560 m (Figure 17E & L). Clusters 5, 8, 9, and 10 were all dominated by either the stalked crinoid Bathycrinus carpenterii or Sabellidae tubes (or both) on soft bottom from 2270 to 3340 m depth, although Cluster 9 consistently had high densities of the sea cucumber Kolga hyalina present (Figure 17F, I, J, & K). The bamboo coral reef found between 935 and 995 m made up cluster 6 (Figure 17G). Cluster 7 was dominated by the anemone c.f. Bathyphellia with tube worms and the glass sponge Asconema megaatrialia present on hard substrate and occurred between 2375 and 3270 m (Figure 17H). In addition to the glass sponge ground, Cluster 12 also was dominated by brittle stars, burrowing bivalves, and scallops between 915 and 1390 m (Figure 17N).

While the cluster analysis identified similar habitat types that were observed during the ROV dives, some habitats were missed due to low sample size (e.g. limited video lines), such as the known Sclerolinum forests in the diffuse venting regions of Ægir’s Spring (which was put into Cluster 11) and the brittle star beds observed in Cluster 12. Other clusters perhaps could be combined into one cluster, such as Clusters 5, 8, 9, and 10. Further annotation of the visual data is needed (and planned).
6.1.2 - Geology
The study areas on this cruise are located on a mid-ocean spreading ridge (Figure 18). The landscape in this area is young, dynamic and dramatic. It is characterised by long (<40 km) and up to 1300 m high mountain ridges, with several peaks. The main ridges are separated by valleys and deep flat-bottomed basins (<3400 m b.s.l.), containing up to tens of meters thick sediment deposits. Their time of deposition is unknown, but may have taken hundreds of thousands, or even millions of years. Along the crest of Mohn’s ridge, the up to 15 km wide rift valley marks the boundary between the North-American and the Eurasian tectonic plates. This is where seafloor spreading is actively taking place.
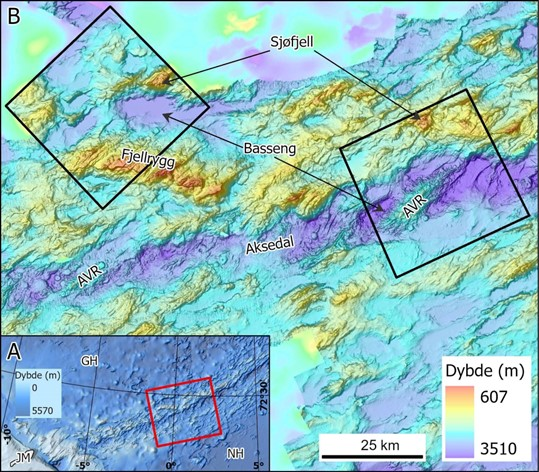
In survey area NH3-B06 there are multiple examples of volcanic activity. The youngest part within the rift valley is a large axial volcanic ridge, which is prominent in bathymetry data, along with volcanic cones, flat-topped volcanos and several fault scarps. In this area we have also observed pillow lava structures and large fissures on video. Evidence of active hydrothermal fields such as bacterial mats, precipitates and hydrothermal chimneys with active outflow were also observed. Warm magma flowing up from the deep along with tectonic processes such as faulting and earthquakes cause seabed displacement, e.g. local uplift of 100s to 1000s meters along the margins of the rift valley. This also leads to repeated mass movement events, recognisable from bathymetry data, and in sub-bottom sediment profiles and video. Survey area NH3-B07 on the other hand is outside the rift valley, and has a slightly simpler and more subdued landscape, although it too is characeterised by high mountain ridges interlain by flat-bottomed basins and valleys.
Generally, a range of geological processes are active in the area and the geodiversity in the area is high. Especially for seabed morphology and geomorphology, but also with respect to substrate, ranging from very fine grain sizes such as Sandy mud to mixed and very coarse grain sizes such as Cobbles and boulders. There is also a lot of Exposed bedrock in the survey areas, primarily on the mountain ridges.
6.2 - Full Stations
Two full stations were completed during the cruise, R3753 (P8) and R7363 (P66). A total of 2 gravity cores for establishing sedimentation rates and genesis, 2 multi corers for chemistry, 1 box corer (0.1 m2) for emerging contaminants (CECs), 10 box corers (0.25 m2) for macrofauna, 2 Agassiz trawls and 2 Beam trawls for epifauna, and 4 RP sleds for hyperbenthos were deployed. However, given the time constraints and weather conditions, only one station of 5 replicates and one station with 2 replicates at box NH3-B03 were sampled.
6.2.1 - R3753 (P8)
The first full station took approximately 54.5 hours over the course of 5 separate days (3, 4, 6, 7, and 10 October), due to weather conditions limiting the deployment of gear or requiring all operations to stop.
Starting on 3 October, a CTD was deployed and collected bottom water for eDNA before the ROV went into the water. After the completion of the video line, in addition to the 2 push corers for geology, the ROV collected chemistry samples with 2 blade corers in modified aluminum frames to compare to the 0.1 m2 box corer sample and 6 push cores (two of these with aluminum liners) to compare to the multi corer samples. Both the blade corer sampling and the push core sampling for chemistry were successful. Additionally, 2 Niskin Bottles mounted on the ROV were fired to collect bottom water for eDNA to compare to the bottom water collected by the CTD; however, due to a mounting error, both Niskin Bottles on the ROV misfired and did not collect any bottom water. The ROV stayed at the bottom as the box corers were deployed to ensure success in the sampling of the drop gear.
The first drop gear that was deployed was the small box core (0.1 m2) rather than the gravity corer due to the weather conditions. Video observation from the ROV showed the box corer bouncing off the seabed due to poor weather conditions before taking the sample when landing again. The sample was presumably disturbed at the surface, but was still taken to be compared to the samples taken with blade corers. Three larger box core (0.25 m2) attempts were then performed. The first 2 casts failed, as the box corer did not release due to technical problems in the release mechanism. On the third attempt, the box corer did not release either, and the ROV had to manually release the box corer mechanism to trigger the spade. However, due to the bad weather picking up and the wave conditions increasing, the box corer bounced twice against the seafloor when landing in the third attempt, massively disturbing the surface. Nonetheless, the sample was processed once upon deck, although eDNA and sediment pigments samples were not taken.
The catch of the Agassiz trawl was very small and was not sieved through the sieving table (Photo 8). Instead, the entire catch was sorted into taxonomic groups and placed in trays and petri dishes and the whole content was preserved. The catch was dominated by sea cucumber Kolga sp. Due to the uncertainty of whether this was a realistic catch with the mesh size (1 cm), it was unclear if the sample was viable or if the deployment failed. Afterwards, the RP sled was deployed and contained only sediment fragments in the net and part of the cod-end connecting apparatus was missing upon retrieval. It was suspected the gear landed incorrectly and that the sampling failed. Sampling then was postponed due to bad weather. During the bad weather window, it was made sure that the release mechanism on the box corer worked properly on deck and adjustments were made to guarantee successful triggers.

On 6 October, when the weather calmed down, we returned to R3753 to continue the physical sampling. The first gravity corer was successfully retrieved and collected 435 cm of sediment (Table 6). Then 2 more casts of the larger box core (0.25 m2) were retrieved successfully, containing mud, foraminifera, polychaeta tubes of Sabellidae family, and a Kolga. Then the multi corer was successfully deployed, although there were signs of disturbance in some of the cores. The longest core (up to 56 cm) had a crack in the middle and was therefore assigned core D. Another core with signs of disturbance (possible sample loss at the bottom of the core) was assigned core C, while the two primary cores for chemistry analyses, A and B, seemed to be intact and of sufficient length (44.5 cm for core A and 35.5 cm for core B). All the cores had seawater above the surface and the multi corer was therefore approved. The results are to be compared to those from push core analysis.
| Core name | Lat (DD) | Long (DD) | Depth (m) | Core Length (cm) | # of Section | Retrieval Date (UTC) | Retrieval Time (UTC) | Comments |
| KPH25-711-GC01 | 72.2996 | 1.8641 | 2669.6 | 435 | 5 | 06.10.2025 | 08:50 | R3753. Core catcher in separate bag. |
| KPH25-711-GC02 | 72.3908 | 1.0846 | 2273.8 | 478 | 5 | 10.10.2025 | 21:03 | R3763. Top 0-4 cm is in separate bag. Core catcher in separate bag. |
Due to the failure of the 1st RP sled, it was decided to re-deploy the RP sled, only for it to come up with the cod-end completely missing. It was also decided to deploy the spare beam trawl due to the uncertainties of the net mesh size on the Agassiz trawl to compare the catch size (Photo 9). The catch from the beam trawl had lots of stones and boulders in it. In fact, the net of the beam trawl ripped apart due to big boulders. In this case, the smaller fraction of the catch containing small stones and gravel was sieved through a 2 mm sieve and a 0.5 mm sieve was placed beneath the main sieve to retain the finer fraction of sediment with fauna, which will be sent to the UiB Museum for further processing, while the big rocks were washed and kept aside until they were checked for animals. Animals found on the rocks were added to the catch when found and then the rocks were discarded. While washing the sample, delicate fauna was separated from the rest of the fauna and sediment, particularly from stones, into a small tray. The catch was composed by Kolga sp., anemones, incl., c.f. Bathyphellia sp., Gastropoda indet. and some polychaeta tubes.

The remaining three larger box core samplings were resumed on 10 October. The first deployment that day happened when there were still considerable waves, and from ROV footage we could see that the box corer landed too fast against the seafloor, and a lot of sediment was flushed out through the top opening doors of the gear. Additionally, when lifted on deck, the box corer slammed against the A-frame several times, disturbing even more the sediment surface. The sample was anyway processed but deemed of bad quality. After that, weather conditions improved, and two more successful box cores with perfect landings and undisturbed sediment surfaces were retrieved. The viable samples contained mud, foraminifera, Sabellidae tubes and some porifera of genus Thenea.
6.2.2 - R3763 (P66)
The second full station took approximately 34.5 hours to complete over the course of 2 continuous days (10 and 11 October).
Starting on 10 October, the CTD was deployed and collected bottom water for eDNA. Then the ROV was deployed for the video line and stayed at the bottom during drop gear deployment. Once the video line was completed with biological and geological sampling, 2 Niskin Bottles mounted on the ROV were successfully fired for eDNA. The gravity corer was successfully deployed and collected 478 cm sediment (Table 6). Then the two larger box core casts were retrieved. The first deployment was successful, however, on the second cast the box core landed very close to the hole made by the previous landing, probably sampling slightly disturbed sediments. The sample was processed, but results should be interpreted carefully. The multi corer was then successfully retrieved, delivering six high quality cores of up to 31 cm length.
Then the 3rd RP sled was deployed, and once again the cod-end was missing upon retrieval, with the RP sled coming up empty. It was then decided to attempt one last time with the RP sled and adjust the towing time to 15 minutes rather than 30 minutes. When the 4th RP sled was retrieved, the net and cod-end was completely filled with sediment consisting of mud and foraminifera, and there were many Kolga and Prosobranchia in the sample, resulting in a long processing and decanting time. The sample was decanted and goldwashed following standard MAREANO procedures, however due to the sediment and Kolga, another decanting was required to attempt to clean the sample as best as possible. This resulted in polychaetes and crustaceans in the decanted fraction and approximately 25 L of foraminifera and other fauna remaining. It is suspected that the RP sled dragged in the sediment while being towed and the wire length may have been too long (at 1.7x the depth), thus scrapping the seafloor and suspending the sediment in front of the sled, completely filling the net with towed epibenthos and sediment rather than purely hyperbenthos.
The beam trawl catch at station R3763 was the largest trawl catch of the cruise. The sample was, therefore, washed over a 5 mm sieve on the sieving table, with the finer fraction collected on a 0.5 mm sieve placed underneath. The number of stones and their size were lower in this samples compared to the previous beam trawl sample. Stones without fauna attached were separated from the samples as much as possible and discarded.
Due to the size of the catch, only partial sorting into major taxa was possible on board. Fragile and rare fauna, and fauna attached to stones were picked from the main catch and were separated into different containers to avoid fauna damage during preservation. Fragile and rare fauna, and fauna attached to stones were picked from the main catch and were separated into different containers to avoid fauna damage during preservation.
Kolga sp. was by far the most abundant taxon. Other common taxa included anemones and poriferans attached to small stones, and molluscs (gastropods and bivalves). This haul captured higher diversity than the other trawl samples, including, e.g., the only trawled sea star (Tylaster sp.), delicate bivalves Hyalopecten sp., wood fragments with possible associated fauna, and pieces of fishing gear overgrown with epifauna. Fish collected in trawl included 15 individuals of Lycodes frigidus and one Paraliparis sp. (Photo 10).

The Agassiz trawl sample at R3763 was relatively small, contained almost no sediment, and included no stones. The sample was washed over a 1 mm sieve for larger organisms, and with a 0.5 mm sieve underneath to collect the finer fraction. The catch was sorted into major taxa on board. Kolga sp. again heavily dominated. Other common taxa included arthropods (mainly decapods), gastropods, poriferans, and polychaeta tubes. The sample also contained fish (eight Lycodes sp. and three Lycodes frigidus) and one cephalopod (Cirroteuthis muelleri) (Photo 11).

6.3 - CTDs
A total of 11 CTDs were deployed in B06 and 2 CTDs in B07. In general, the top layer was made up of warm water typically greater than 4°C, where the stations from NH3-B06 were between 6 and 10°C, and NH3-B07 were cooler (T<5°C). The salinity at the surface fluctuated between the stations, generally remaining between 34.5 and 35.2 ppt, although the stations in NH3-B07 (S = 34.55 to 34.65) were generally fresher than the stations in NH3-B06 (S = 34.8 – 35.1). At approximately 50 m, there was a spike in salinity to around 35 ppt before steadily decreasing. The thermocline and halocline observed were between 50 and 400 m. The water temperature continued to slowly decline before stabilizing at -0.5°C around 1500 m. Salinity slowly increased before stabilizing at around 34.94 ppt at 2000 m. There were elevated dissolved oxygen concentrations observed between 50 to 500m before it steadily reduced with increasing water depth.
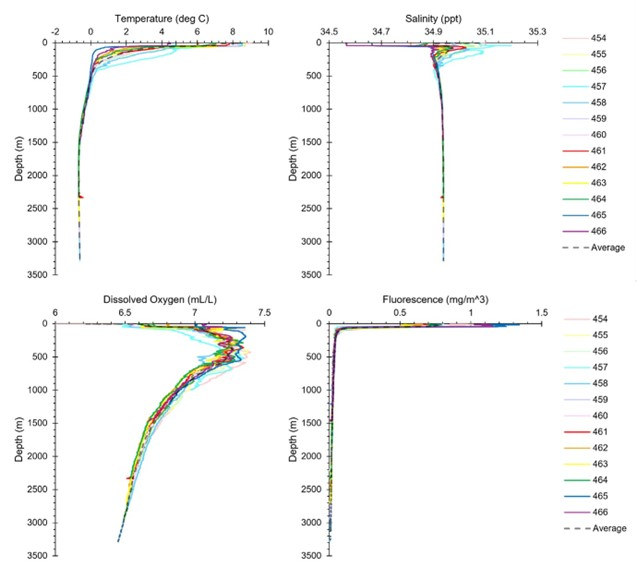
Some patterns that deviated from the average were observed at some of the stations. The thermocline and halocline for R3747 (CTD457) was generally more pronounced compared to the other stations in NH3-B06 where warmer and more saline water was present in the upper 500 m. The elevated dissolved oxygen concentrations at R3747 were observed at 500 m and 700 m. At R3756 (CTD 461), or Ægir’s Spring, there was a spike in temperature and drop in both salinity and dissolved oxygen observed in the profiles at approximately 2300 m (where the vent field is).
It must be stated however that the CTDs only capture a single time point of data and likely do not reflect the entirety of the oceanographic parameters at the stations. More standardized replicates over a time series and well planned CTD transects are required for a more thorough evaluation of the oceanographic conditions in the region.
7 - Station Summaries
Table 7. Descriptive summary of the stations surveyed during 2025007011 with a reference picture by ROV Ægir6000 (NORMAR) included.
| Station | P# | Box | VL & Dive Duration (Hours) | Start Depth (m) | Activities | ROV Samples | Short Summary | Representative Picture by ROV Ægir6000 (NORMAR) |
| R3740VL3831 | P14 | NH3-06 | 3:25 & 5:30 | 1669 | Multibeam SBP CTD ROV | 2 biology 2 geology | Video line consisting of sandy mud with agglutinated foraminifera and Neohela burrows. Neohela and actiniaria present throughout. |  Center-20250926134121.jpeg. Center-20250926134121.jpeg. |
| R3741VL3832 | P70 | NH3-06 | 4:05 & 5:40 | 1825 | Multibeam SBP ROV | 5 biology 2 geology | Video line switching between sandy mud, exposed bedrock, and pillow lava. Mainly soft bottom with tetractinellida and polymastida sponges present and patches of spicule bottom. |  Center-20250928095329.jpeg. Center-20250928095329.jpeg. |
| R3742VL3833 | P15 | NH3-06 | 3:00 & 3:50 | 1825 | CTD ROV | 2 biology 2 geology | Video line switching between exposed bedrock, muddy sand, and gravelly muddy sand. High density of rossellidae sponges and bryozoans on the exposed bedrock wall. Subsea transit to P13. |  Center-20250928164316.jpeg Center-20250928164316.jpeg |
| R3743VL3834 | P13 | NH3-06 | 3:40 & 4:20 | 1453 | ROV | 1 biology 3 geology | Video line consisting of gravelly sandy mud with agglutinated foraminifera and Neohela burrows. Neohela sp. and actiniaria present throughout with patches of rocky outcrops and spicule mats dominated by sponges. |  Center-20250928205610.jpeg Center-20250928205610.jpeg |
| R3744VL3835 | P81 | NH3-06 | 3:30 & 4:35 | 2211 | Multibeam SBP CTD ROV | 4 biology 4 geology | Video line consisting of gravelly sandy mud with some agglutinated foraminifera and patches of exposed bedrock. Stalked crinoid Bathycrinus carpenterii, actiniaria and some Neohela sp. present with patches of sponges dominating exposed bedrock. Subsea transit to P81b. |  Center-20250929080044.jpeg Center-20250929080044.jpeg |
| R3744VL3836 | P81b | NH3-06 | 2:55 & 5:00 | 2010 | ROV | 2 biology 2 geology | Video line consisting of gravelly sand and gravelly sandy mud with some agglutinated and calcareous foraminifera then transitioning to a steep wall of pillow lava. Steep wall dominated by tetractinellida and polymastida sponges. Subsea transit to P16. |  Center-20250929111259.jpeg. Center-20250929111259.jpeg. |
| R3745VL3837 | P16 | NH3-06 | 2:35 & 3:55 | 2826 | ROV | 2 biology 3 geology | Video line consisting of muddy sand all the way with lots of lebensspuren. Video line dominated by Bathycrinus carpenterii. Subsea transit to P17. |  Center-20250929165534.jpeg. Center-20250929165534.jpeg. |
| R3746VL3838 | P17 | NH3-06 | 2:45 & 4:00 | 2474 | ROV | 3 biology 1 geology | Video line consisting of sandy mud, mud and sand with gravel, cobbles, and boulders, and exposed bedrock. Soft bottom dominated by Bathycrinus carpenterii and hard bottom dominated by sponges and unstalked crinoids of Antedonoidea family. |  Center-20250929215137.jpeg. Center-20250929215137.jpeg. |
| R3747VL3839 | P88 | NH3-06 | 2:50 & 3:55 | 998 | Multibeam SBP CTD ROV | 3 biology 2 geology | Video line consisting of exposed bedrock and biogenic coverage. Video line dominated by bamboo coral and bryozoans with some sponges, tunicates, and other associated fauna. |  Center-20250930062113.jpeg. Center-20250930062113.jpeg. |
| R3748VL3840 | P87 | NH3-06 | 4:55 & 7:10 | 3236 | Multibeam SBP CTD ROV | 1 biology 3 geology | Video line consisting of pillow lava with mud and sandy mud. Video line dominated by c.f. Bathyphellia sp. and sabellidae tubes. |  Center-20250930153132.jpeg. Center-20250930153132.jpeg. |
| R3749VL3841 | P1 | NH3-06 | 2:50 & 4:20 | 3091 | ROV | 3 biology 2 geology | Video line consisting of sandy mud and lebensspuren. Video line dominated by Bathycrinus carpenterii and sabellidae tubes, with some Thenea sp. and Elpidia sp. Subsea transit to P7. |  Center-20250930234534.jpeg. Center-20250930234534.jpeg. |
| R3750VL3842 | P7 | NH3-06 | 3:05 & 5:50 | 3198 | ROV | 2 biology 2 geology | Video line consisting of sandy mud and lebensspuren. Video line dominated by Bathycrinus carpenterii and sabellidae tubes, with some c.f. Bathyphellia sp., Thenea sp. and Elpidia sp. and patches of biogenic debris. |  Center-20251001050609.jpeg Center-20251001050609.jpeg |
| R3751VL3843 | P86 | NH3-06 | 03:05 & 5:50 | 3334 | ROV | 2 geology | Video line consisting of mud with biogenic debris. Video line dominated by sabellidae tubes and amphipods, with some Caulophacus arcticus. |  Center-20251001105505.jpeg. Center-20251001105505.jpeg. |
| R3752 | P3 | NH3-06 | 2900 | CTD | Delay of station due to adverse weather. | |||
| R3753VL3844 | P8 | NH3-06 | 5:55 & 9:05 | 2677 | Full Station: Multibeam SBP CTD ROV Box Corer (0.1 m2) Box Corer (0.25 m2) Agassiz Trawl RP Sled | 1 CTD – bottom water for eDNA 2 ROV biology 8 ROV chemistry 2 ROV geology 1 Box Corer (0.1 m2) chemistry 2 Box Corers (0.25 m2) biology – failed 1 Agassiz Trawl biology 1 RP Sled biology – failed. | Video line consisting of sandy mud with calcareous foraminifera and lebensspuren. Video line dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp. and c.f. Bathyphellia sp. ROV stayed at bottom during gear deployment. Had to abort the full station early due to weather. Box corers for biology hit the bottom of the seafloor due to waves and were not viable. Agassiz trawl catch was small. RP Sled had nothing in the cod-end. Multicorer, Gravity Corer, and additional Box Corers were delayed due to weather. |  Center-20251003131609.jpeg. Center-20251003131609.jpeg. |
| R3754VL3845 | P68 | NH3-06 | 4:15 & 6:45 | 2673 | ROV | 3 biology 3 geology | Video line consisting of sandy mud, muddy sand, and exposed bedrock with lebensspuren and calcareous foraminifera all the way. Soft bottom regions dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., and c.f. Bathyphellia sp.. Bedrock dominated by sponges and ascidians. |  Center-20251004131348.jpeg. Center-20251004131348.jpeg. |
| R3752VL3846 | P3 | NH3-06 | 2:45 & 5:55 | 2900 | ROV | 1 biology 3 geology | Video line consisting of sandy mud, gravelly sandy mud, pillow lava and exposed bedrock with several deep crevices. Soft bottom regions dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., c.f. Bathyphellia sp. and other actiniaria. |  Center-20251004203654.jpeg. Center-20251004203654.jpeg. |
| R3755VL3847 | P69 | NH3-06 | 2:35 & 6:25 | 2638 | ROV | 1 biology 2 geology | Video line consisting of sandy mud with lebensspuren and calcareous foraminifera. Video line dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., c.f. Bathyphellia sp. and other actiniaria. Recorded the top camera on ascent by external request. |  Center-20251005035432.jpeg. Center-20251005035432.jpeg. |
| R3756VL3848 | P82a | NH3-06 | 4:25 & 6:15 | 2308 | Multibeam SBP CTD ROV | 1 CTD – bottom water for eDNA 2 biology 2 geology | Video line started at the vent field and contained bacterial mats, pillow lava, and muddy sand then it transitioned outside of the venting area to muddy sand and pillow lava/exposed bedrock. Video line contained a siboglinid tubeworm Sclerolinum contortium, Bathycrinus carpenterii, and Antedonoidea then transitioned to sponge domination on the exposed bedrock. 2 blade corer samples of Sclerolinum contortium were collected for an external party. Subsea transit to P82b. |  Center-20251005134850.jpeg. Center-20251005134850.jpeg. |
| R3757VL3849 | P82b | NH3-06 | 5:40 & 7:30 | 2330 | ROV | 4 biology 10 geology | Video line containing mostly pillow lava and exposed bedrock with some patches of sandy mud and muddy sand. Video line contained unstalked crinoids and sponges on exposed bedrock. An additional video was recorded for exploration only. Subsea transit to 82c. |  Center-20251005201215.jpeg. Center-20251005201215.jpeg. |
| R3758VL3850 | P82c | NH3-06 | 2:35 & 5:30 | 2432 | ROV | 1 geology | Video line containing mostly pillow lava, lava tubes, and exposed bedrock. Video line contained sponges, unstalked crinoids, ascidians, and Actiniaria. Recorded the top camera on ascent by external request. |  Center-20251006024215.jpeg. Center-20251006024215.jpeg. |
| R3753 | P8 | NH3-06 | 2677 | Full Station: Gravity Corer Multi Corer Box Corer (0.25 m2) Beam Trawl RP Sled | 1 Gravity Corer geology 1 Multi Corer chemistry 2 Box Corer (0.25 m2) biology 1 Beam Trawl biology 1 RP Sled biology – failed 2 ROV niskin bottles for eDNA – failed | Continued with the full station sampling. Due to uncertainty in the quality of the Agassiz Trawl catch since it was small, a Beam Trawl was also done and came up muddy and the net was damaged by rocks. The RP Sled came up empty and was missing the cod-end. Had to stop due to weather. ROV stayed down at bottom during the drop gear sampling. | ||
| R3759VL3851 | P10 | NH3-06 | 2:55 & 6:20 | 2423 | Multibeam SBP CTD ROV | 4 biology 2 geology | Video line containing sandy mud/muddy sand with calcareous foraminifera. Video line dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., c.f. Bathyphellia sp. and other actiniaria. There were patches of dead Pourtalesia shells throughout. |  Center-20251009001930.jpeg Center-20251009001930.jpeg |
| R3760VL3852 | P9 | NH3-06 | 3:20 & 6:00 | 2665 | Multibeam SBP CTD ROV | 3 biology 2 geology | Video line containing sandy mud with lebensspuren. Video line dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., c.f. Bathyphellia sp. and other actiniaria. Two copper lines were observed on the seafloor. |  Center-20251009101646.jpeg. Center-20251009101646.jpeg. |
| R3761VL3853 | P84 | NH3-06 | 2:50 & 4:45 | 2453 | ROV | 2 geology | Video line containing sandy mud with patches of pillow lava at the start and signs of slides. Soft bottom dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., c.f. Bathyphellia sp. and other actiniaria. Hard bottom dominated by sponges. |  Center-20251009172731.jpeg. Center-20251009172731.jpeg. |
| R3762VL3854 | P85 | NH3-06 | 2:25 & 4:35 | 2531 | ROV | 1 biology 2 geology | Video line containing sandy mud/muddy sand with calcareous foraminifera and lebensspuren then transitioning to exposed bedrock and pillow lava with fissures. Soft bottom dominated by sabellidae tubes, crinoids, anemones, and Thenea sp. Kolga sp. present in variable densities. Hard substrate dominated by sponges and crinoids. |  Center-20251010003732.jpeg. Center-20251010003732.jpeg. |
| R3753 | P8 | NH3-06 | 2677 | Full Station: Box Corer (0.25 m2) | 3 Box Corer (0.25 m2) biology | Continued with the full station sampling. Box Corers contained mud, foraminifera and some polychaeta tubes. ROV stayed down at bottom during the drop gear sampling. | ||
| R3763VL3855 | P66 | NH3-06 | 3:15 & 3:55 | 2251 | Full Station: Multibeam SBP CTD ROV Gravity Corer Multi Corer Box Corer (0.25 m2) RP Sled Beam Trawl Agassiz Trawl | 1 CTD – bottom water for eDNA 2 ROV biology 2 ROV Niskin bottles for eDNA 2 ROV geology 1 Gravity Corer geology 1 Multi Corer chemistry 2 Box Corers (0.25 m2) biology 2 RP Sled biology – 1 failed 1 Beam Trawl biology 1 Agassiz Trawl biology | Video line consisting of sandy mud with calcareous foraminifera and lebensspuren. Video line dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Neohela burrows. ROV at the bottom during drop gear deployment. All drop gear were deployed successfully, 1st RP sled lost the cod-end so a second one was deployed with a shorter towing time (15 minutes) and came back with mud, foraminifera, epifauna and hyperbenthos. Due to a miscommunication, the Beam Trawl was deployed before the Agassiz Trawl, both had a viable catch. |  Center-20251010180950.jpeg. Center-20251010180950.jpeg. |
| R3764VL3856 | P4 | NH3-06 | 2:25 & 4:35 | 2425 | ROV | 1 biology 2 geology | Video line containing sandy mud with calcareous foraminifera and lebensspuren. Soft bottom dominated by Kolga sp., Bathycrinus carpenterii, and sabellidae tubes, with some Thenea sp., Pourtalesia sp., c.f. Bathyphellia sp. and other actiniaria. Small patches of Neohela aggregations. |  Center-20251012035913.jpeg. Center-20251012035913.jpeg. |
| R3765VL3857 | P67 | NH3-06 | 3:25 & 4:50 | 3080 | ROV | 5 geology | Video line containing variable substrate from steep slopes with cobbles and boulders to exposed bedrock to gravelly sandy mud to sandy mud. Soft bottom dominated by Bathycrinus carpenterii, some sponges, crinoids, and anemones on hard substrate. |  Center-20251012083300.jpeg Center-20251012083300.jpeg |
| R3766VL3858 | P6 | NH3-06 | 2:55 & 4:30 | 3269 | ROV | 1 biology 2 geology | Video line containing sandy mud with patches of exposed bedrock and biogenic debris (dead sponges) and calcareous foraminifera. Bathycrinus sp. and Sabellidae tubes dominated the soft bottom. Large glass sponges (Caulophacus arcticus and Asconema megaatralia) present on hard substrate. Subsea transit to P11. |  Center-20251012164200.jpeg. Center-20251012164200.jpeg. |
| R3767VL3859 | P11 | NH3-06 | 1:15 & 2:30 | 2610 | ROV | 1 geology | Video line mainly with exposed bedrock of pillow lava with varying sediment coverage and soft sediment patches. Both soft bottom and hard bottom dominated by crinoids. Porifera also present on hard substrate. Dive aborted due to weather. |  Center-20251012200741.jpeg. Center-20251012200741.jpeg. |
| R3768VL3860 | P2 | NH3-06 | 2:45 & 5:10 | 2929 | ROV | 2 biology 2 geology | Video line with mainly sandy mud with lebensspuren and patches of compacted sediment. Soft bottom dominated by Sabellidae, Elpidia sp., and Bathycrinus carpenterii with some Thenea sp. and actiniaria. Kolga appeared at the end of the dive. |  Center-20251013035007.jpeg Center-20251013035007.jpeg |
| R3769VL3861 | P5 | NH3-06 | 2:30 & 4:25 | 3094 | ROV | 4 geology | Video line with mainly sandy mud with lebensspuren and patches of pillow lava. Soft bottom dominated by Bathycrinus carpenterii and actiniaria with some patches of dead Asconema megaatralia. |  Center-20251013113801.jpeg. Center-20251013113801.jpeg. |
| R3767VL3862 | P11 | NH3-06 | 2:05 & 4:20 | 2545 | ROV | 1 biology 3 geology | Video line with mainly exposed bedrock of pillow lava and tubular flows and patches of sandy mud. Soft bottom dominated by crinoids and hard bottom dominated by crinoids and porifera. Continued where dive stopped when aborted. |  Center-20251013165351.jpeg Center-20251013165351.jpeg |
| R3770VL3863 | P83 | NH3-06 | 3:15 & 5:20 | 1960 | ROV | 2 biology 2 geology | Video line mainly muddy sand with calcareous and agglutinated foraminifera and lebensspuren, and some patches of exposed bedrock. There were several fluid escape features and depressions on the seabed. Soft bottom dominated by crinoids, anemones, Sabellidae tubes, and crustaceans like amphipods, Neohela, and Bythocaris. Hard bottom dominated by tetractinellida and polymastida sponges. |  Center-20251014151452.jpeg. Center-20251014151452.jpeg. |
| R3771VL3864 | P12 | NH3-06 | 2:55 & 4:00 | 2140 | Multibeam SBP ROV | 1 biology 2 geology | Video line with mainly muddy sand and gravelly muddy sand with lebensspuren and 80% cover of calcareous and agglutinated foraminifera. The beginning of the video line was dominated by Kolga, then stalked crinoids, anemones, Sabellidae tubes, and Neohela dominated the line. |  Center-20251014191359.jpeg. Center-20251014191359.jpeg. |
| R3772VL3865 | P90a | NH3-07 | 4:50 & 6:35 | 1263 | Multibeam SBP CTD ROV | 1 CTD – bottom water for eDNA 8 biology 4 geology | Video line consisted mainly of bedrock with patches of muddy sand, muddy sandy gravel, gravelly muddy sand, or spicule mat. Soft bottom dominated by brittle stars and bivalves and hard bottom dominated by glass sponges, unstalked crinoids, and soft corals. |  Center-20251015084422.jpeg. Center-20251015084422.jpeg. |
| R3773VL3866 | P90b | NH3-07 | 3:30 & 3:45 | 1272 | ROV | 6 biology 2 geology | Video line consisted mainly of gravelly muddy sand with patches of spicule mat, with some areas containing lots of agglutinated foraminifera. There were some fluid escape features that contained egg-like masses. Soft bottom dominated by brittle stars and bivalves and hard bottom dominated by glass sponges, unstalked crinoids, and soft corals. Numerous fish Gaidropsarus argentatus were observed near the glass sponges. Subsea transit to P90c. |  Center-20251015180224.jpeg. Center-20251015180224.jpeg. |
| R3774VL3867 | P90c | NH3-07 | 3:10 & 4:05 | 1462 | ROV | 1 biology 1 geology | Video line with mainly exposed bedrock with patches of biogenic debris like spicule mat. Glass sponges, encrusting sponges, carnivorous sponges, brittle stars, bryozoans, and bivalves dominated the video line. |  Center-20251015225905.jpeg. Center-20251015225905.jpeg. |
| R3775VL3868 | P20 | NH3-07 | 2:40 & 5:20 | 2519 | ROV | 1 biology 1 geology | Video line with mainly muddy sand with lebensspuren and foraminifera all the way. Area dominated by Bathycrinus and anemones, with Kolga becoming more present at the end. |  Center-20251016053144.jpeg. Center-20251016053144.jpeg. |
| R3776VL3869 | P29 | NH3-07 | 2:50 & 3:40 | 1461 | Multibeam SBP CTD ROV | 1 CTD – bottom water for eDNA 2 biology 2 geology | Video line with mainly muddy sand and gravelly muddy sand with a lot of agglutinated foraminifera and patches of exposed bedrock. Soft bottom dominated by brittle stars and anemones, spicule and hard bottom dominated by Geodia, Stelletta and other tetractinellida and polymastida sponges. |  Center-20251016142043.jpeg. Center-20251016142043.jpeg. |
| R3777VL3870 | P30 | NH3-07 | 2:25 & 3:20 | 1485 | ROV | 2 biology 1 geology | Video line mainly consisted of muddy sand and gravelly muddy sand with agglutinated foraminifera and spicule mat. Spicule mat dominated by Geodia and Stelletta and soft bottom dominated by anemones and brittle stars. |  Center-20251016184447.jpeg Center-20251016184447.jpeg |
| R3778VL3871 | P71 | NH3-07 | 4:30 & 5:50 | 1639 | ROV | 2 biology 3 geology | Video line started with muddy sand then transitioned to exposed bedrock with patches of spicule mat covering the bedrock areas. Agglutinated foraminifera present on the muddy sand. Hard bottom and spicule mat dominated by Geodia, Stelletta, and glass sponges and soft bottom covered with anemones. An additional transect was conducted to reach the top. |  Center-20251017012602.jpeg. Center-20251017012602.jpeg. |
| R3779VL3872 | P71b | NH3-07 | 5:00 & 6:00 | 1145 | ROV | 2 biology 2 geology | Video line with mainly gravelly muddy sand and muddy sandy gravel with agglutinated and calcareous foraminifera and areas of exposed bedrock. Soft bottom dominated by brittle stars and sabellid worms, with patches of Keratoisididae coral on biogenic substrate. Hard bottom dominated by glass sponges. Conducted an exploratory section at the end of the video line and found more Keratoisididae aggregations. |  Center-20251017052336.jpeg Center-20251017052336.jpeg |
8 - Limitations
MAREANO cruise 2025007011 to Mohn’s Ridge encountered numerous difficulties due to ship and equipment limitations and weather conditions, which ultimately impacted our ability to complete all 3 boxes as planned (NH3-B06, B07, B08). Regardless of those difficulties, it was unrealistic to expect to finish all 3 planned boxes and expectations need to be adjusted for future cruises to the deep sea. Work at the deep sea is considerably more time consuming and that needs to be clearly communicated. The following sections go into more detail about the limitations encountered on this cruise.
8.1 - Mobilization time and Demobilization time
ROV Ægir requires 24 hours minimum for mobilization and roughly 12 hours for demobilization, where the ship needs to be stationed near shore after the ROV is loaded onboard.
We could not start mobilization until the 23 September because Research Vessel Kronprins Haakon was anchored and not at the dock until the morning of 23 September.
There were 2 more cruises following 2025007011 that will use the ROV Ægir6000, with 1 cruise between that will not. Due to this cruise time allocation, we must completely demobilize Ægir6000 so the cruise between 2025007011 and 2025007013 can use the moonpool. This means we must return to port 1 day earlier than planned (19 October rather than 20 October) to demobilize Ægir6000 as well as MAREANO equipment.
We lost 1.5 days of this cruise to mobilization and demobilization time.
8.2 - Weather conditions
Mohn’s Ridge and the Norwegian Sea is prone to adverse weather conditions from September to November, making it not an optimal timeframe for MAREANO cruises. MAREANO cruises require conditions that allow operating an ROV and traditional sampling gear (gravity corer, multicorer, box corer, trawl, and sled) to be able to collect the data necessary for fulfilling our deliverables. Due to the nature of the open ocean, when adverse weather comes there is no place to shelter and the ship must stop all operations and wait.
In addition, when the weather is bad, the ROV must take longer to ascend and descend to account for the heave of the swell. On normal conditions, the ROV can go up to 0.8 knots for ascending and descending. During bad weather, the ROV may need to reduce the speed to 0.4 knots to reduce risking damage to the winch.
Kronprins Haakon’s working deck is very exposed, making it dangerous to operate drop gear (e.g. gravity corer, multicorer, box corer) in adverse weather conditions. This means full station operations cannot be completed in adverse weather conditions. When we did deploy any drop gear in remotely bad weather, the sampling failed due to the gear hitting the seafloor upon deployment or hitting the ship when retrieving it out of water (see more below).
We lost 5 days due to adverse weather conditions prohibiting gear deployment, especially drop gear during full stations.
8.3 - Equipment failure
Due to engine problems slowing our speed, it took 52 hours rather than the estimated 42 hours to arrive to our first station.
R/V Kronprins Haakon also does not have a heave compensator on the A-frame to dampen the impacts of the waves when the weather is bad. This has affected the quality of our physical sampling drop gear (gravity corer, multicorer and box corer) where the gear has hit the bottom multiple times due to the swell despite the lowering speed being reduced at 50 to 100 m before reaching the bottom. The box corer also hit the A-frame of the ship when lifting it out of water, thus disturbing the surface layer. This caused our samples to be unusable and required more replicates to get a viable sample. This is critical for the correct deployment of the box corer and the multi corer and should be urgently considered to be fixed for next cruises on board Kronprins Haakon.
We lost 10 hours due to engine problems.
We lost 6 hours due to drop gear failure due to weather.
8.4 - Towed gear failure
In the Deep-Sea Strategy, we planned to acquire an Agassiz Trawl to collect the megafauna and a Brenke Sled to collect the hyper- and epibenthos at the full stations. These gear types are suited for the depths that are within the survey area and can handle variable terrain conditions, unlike the traditional MAREANO towed gear – RP Sled and Beam Trawl. Despite attempts to acquire the proposed gear, we failed to find an available Brenke Sled to rent and were uncertain on the condition and mesh size of the Agassiz Trawl, as it was unknown and could not be checked by the renter – University of Bergen. Therefore, we brought three MAREANO RP Sleds for surveying the hyperbenthos and two MAREANO Beam Trawls as a backup in case the Agassiz was inadequate and could not capture the smaller megabenthos.
It was not realized until we were on board that we lacked the appropriate depth rated scanmar and floaters (for the RP Sled) to be attached to the geared equipment, therefore it was not possible to track the true lengths and time of the towed gear at the bottom.
8.4.1 - Agassiz trawl and Beam trawl
We measured the inner mesh size of the Agassiz Trawl to be 1 cm once we were onboard, which was larger than MAREANO’s Beam Trawl (5 mm). After the first Agassiz Trawl at P8, it was clear all epifauna smaller than 1 cm were not captured by the Agassiz Trawl and the catch size was small. We made the decision to use the Beam Trawl afterwards to see if we would get a comparable catch size, however the Beam Trawl was filled with mud, rocks and big boulders once it came onboard, indicating the seafloor was too heterogeneous and the mesh size captures lots of sediment and rocks.
When at the second full station, P66, we intended to deploy the Agassiz Trawl first, then deploy the Beam Trawl in case the Agassiz Trawl catch was inadequate and, if time allowed, have a comparison between gear types to test the suitability of the Beam Trawl in the deep sea in order to make a decision to not include the Beam Trawl for future surveys in the region. However, due to miscommunication on deck, the Beam Trawl was deployed before the Agassiz Trawl. The catch on the second Beam Trawl was more viable than the first Beam Trawl at P8. The second Agassiz trawl had a smaller catch than the Beam Trawl and only captured epifauna larger than 1 cm, though it did contain some overlapping species found in the Beam Trawl.
It is recommended that the Beam Trawl is only brought on the next cruise on the condition that a Brenke Sled is also brought to allow a full comparison of the diversity caught between the three gear types (Agassiz Trawl with a modified mesh size, Beam Trawl, and Brenke Sled).
8.4.2 - RP sled
At the first full station, P8, the first RP Sled had very little in the cod-end indicating it may have landed incorrectly. Therefore, we decided to attempt a second RP Sled when we returned to P8 to complete the station. However, the cod-end was missing once the second RP Sled came back on deck, resulting in an empty catch.
At the second full station, P66, we attempted the third RP Sled at the standard towing time (30 minutes), and once again, the replacement cod-end was missing once it came on deck and the RP Sled was empty. We attempted a fourth RP Sled and adjusted the towing time to 15 minutes instead. When the RP came up on deck, the cod-end was filled with foraminifera, mud, and epibenthos, indicating that it had dragged along the seafloor rather than “glided” as it was meant to.
It is recommended that the RP Sled is not used for future surveys in the deep sea and only brought as a back-up with appropriate scan mar and floaters.
We lost 20 hours due to inadequate towing gear not suited for the deep sea.
8.5 - Depth
Operating in the deep sea takes much longer for operations to take place and that must be considered when planning future cruises. Under normal conditions, gear can be lowered between 0.5 and 0.8 knots, depending on the gear type. When the weather is bad, the gear (like ROV) needs to be lowered at half the speed (0.4 knots) for safe deployment. Deployment and retrieval are the most dangerous times for the ROV and drop gear, and conditions need to be appropriate for them to operate safely and be of good quality.
Dives at 1500 m to 2000 m took approximately 4 hours to complete (from leaving deck to back on deck), where ascending and descending takes approximately 1 hour combined. Dives at 2000 to 3000 m took approximately 5 to 6 hours to complete, where ascending and descending takes approximately 2 hours combined. The video lines (800 m long with 4 x 200m long transects) take approximately 2.5 hours to complete on average with standard amount of sampling (3 biological samples per VL line and 2 push cores for geology). When more sampling is done on a VL, the VL can take approximately 3 to 4 hours to complete.
9 - Suggestions for Future Cruises
9.1 - Station Planning
MAREANO station planning (using GRTS) ensures spatially balanced random samples are created. Coupled with a stratification of selected environmental variables (resampled to 50 m resolution), this approach naturally places more samples in strata with more environmental variability and greater (planar) area than in flat, homogenous areas. Video lines of 800 m (planar) will cover considerably more than 800 m distance over ground in steep terrain, while in flat-moderately sloping terrain this effect is negligible. This, together with the fact that the video data are needed to ground-truth a sufficient number of pixels in gridded multibeam data, for onward interpretation and modelling, were discussed at pre-cruise station planning meetings, but with a consensus to adapt as necessary during the cruise. We therefore started the cruise with an open mind on shortening selected video lines as we gained a realistic estimate of ROV-based survey time in this rugged terrain. During the cruise, it quickly became clear that the effects of the sloping and variable terrain (including fine-scale variations not captured in the multibeam bathymetry) were having significant impact on the survey time and resulting in very long video lines (>>800 m) in terms of the distance over ground. We started to adjust the length of the lines that covered considerable depth differences before each dive, however this was an ad-hoc solution for each video line, to bring the length closer to 800 m over ground while keeping it long enough for effective ground-truthing.
For future cruises we suggest a more systematic approach is adopted for shortening video lines in steep terrain, and that this is incorporated in pre-cruise survey planning routines. Several approaches may be adopted but systematic calculation of the distance over ground can be incorporated, optionally with profile views of each survey line, seem to offer a good solution. A formula can then be developed to shorten video line lengths based on slope values and variation, to an agreed minimum (planar) length (e.g. 400 m) matched to the resolution and quality of the multibeam (bathymetry and backscatter) data in a given area (i.e. what is needed for ground truthing and onward use in 2D map products), and experience based estimates of dive time. Whilst some modification during the cruise may still be needed in certain cases, (e.g. if the direction is altered for practical reasons) this pre-planned adaptation will provide a more realistic and time-efficient starting point for those on board and minimize the need for ad-hoc planning. This approach to line length adaptations can be applied equally to GRTS and targeted lines, although the latter could be planned from the outset, to ensure the features of particular interest are retained.
9.2 - Refuge Boxes
During this cruise the reserve boxes were primarily defined on the basis of priority. Due to their proximity to the priority boxes on this cruise, they are not suitable as refuge boxes in case of bad weather. It is highly suggested that in the future refuge boxes are planned in addition to reserve boxes, in case it is necessary to seek shelter, and that these are placed farther away from the priority area, e.g. closer to port and/or closer to Jan Mayen.
9.3 - Full Crew
Due to the changes in workflow, short turnaround between stations and now the possibility of collecting samples with the ROV, which requires people to process them as quickly as possible, it is suggested that for cruises on AMOR, we have a full ship with 4 extra biologists on board. The current workload on the deep-sea cruise is not sustainable with the MAREANO standards of 3 biologists per shift. We would often be diving during mealtimes or processing samples as the ROV was descending and when it arrived at the bottom, biologists were still occupied with tasks down at the lab. Extra biologists would help with the workload and ensure opportunities for switching loggers during the shift since continuous logging fauna in highly biodiverse regions while staring at a screen for extended periods of time can be mentally and physically demanding.
9.4 - Seabed Field Observer (SFO)
Further development into the updated version of SFO is needed to improve the efficiency of logging and processing the annotations made during the video line.
-
Ability to rearrange the order of the buttons
-
Ability to set colors of the buttons
-
Ability to set favorites that appear at the top of the annotation list
-
Ability to see which annotations have been made during a session by all the users
-
Order of the files needs to be sortable by file name or date, it is currently ordered randomly
-
Button order needs to be sortable by group (e.g. Phylum), alphabetical, most used, object ID
-
Ability to log multiple habitats or seabeds at a time for interval logging
-
A narrow top line with e.g. seabed, habitat, that does not disappear when scrolling down.
-
Ability to use shortcuts to e.g. add quantities to a button, add comments, seabed types and seabed features
-
Ability to change session list or template while in a session
-
Ability to automatically write a comment when the comment field opens (instead of having to click on the input field first) and to get suggestions from previous comments
9.5 - Video and Data Processing
Data processing can be significantly streamlined to further minimize the manual input error rate in the archiving of the data during and after the cruise as well as facilitate the rapid delivery of preliminary data products. The current ad-hoc protocol used in this cruise will be formalized in parallel with the discussions involving the developers of SFO so that procedures and scripts can be further refined and ensure the needs of all the cruise participants are covered. Furthermore, some of the tasks related to data management (e.g., video files and renaming) could be automated to save time and ensure a strict consistency.
A clearer definition of the exact level of details required during the live annotation will let the staff better manage their time and leave breathing room in time when processing physical samples requires part of the shift to be in the laboratory.
There is a need for consistent laser point distances, especially when using the same video platform. For this cruise, the laser points were set at 9 cm and could not be adjusted to MAREANO’s standard 10 cm. This is inconsistent with previous cruises with Ægir6000, where the former (red) laser points distances ranged between 15 to 17 cm, although hopefully the laser bracket is more stable and will not change in the future. Therefore, it is even more important than before to note the lasers distance for every dive in the logging sheets to ensure good documentation. Ideally the lasers distances with Ægir6000 will not change much with a stable laser bracket, but to maintain good practice, always note the laser distances.
9.6 - Equipment Wishlist
It is important to consider the type of equipment that would be most useful for future work in the region. Therefore, we propose a “wish list” to keep in mind for the future.
9.6.1 - ROV Gear
Given that a lot of visited areas with the ROV were not suitable to deploy traditional gear to sample biology (i.e. box corer) due to the heterogeneous terrain or unsuitable conditions, quantitative biological samples were only retrieved at the two full stations (with the exception of some blade corers). As most tools that come with Ægir6000 for sample biology are not-quantitative, we propose to develop some tools that would allow us to sample more quantitatively macrofauna at these sites and in a more standardized way than with the current options (having in mind limitations when it comes to sampling area and replication effort).
In addition to the need for quantitative sampling gear, our ROV wish list gear include:
-
ROV-operated box corer
-
ROV-operated grab
-
ROV scraper or scoop
-
Removable and modular sample storage boxes with lids
-
Modified ROV skuff to fit blade corers (to be able to bring more blade corers)
-
Niskin Bottle Stand for ROV Niskin Bottles
-
Push corer holster/holder on the skuff to mount multiple push corers
9.6.2 - Ship or Physical Gear
-
Deep-sea bouys/floaters for towed gear
-
Trawl sensors for towed gear
-
Heave compensator for A-Frame on the back of R/V Kronprins Haakon
-
Brenke Sled
-
Modified mesh size (5 mm) for Agassiz Trawl
10 - Data availability
The following data is available upon request.
In addition to Sub-bottom profiler (SBP) data collected during dedicated MBES surveys, SBP data are also collected between stations on dedicated sampling cruises. These data are shared within one month after the cruise via a file-sharing solution upon request to marinedata@ngu.no. For more information on datasets and map services for Acoustic Seabed Data, see: NGU’s Map Catalogue | Mareano – gathering knowledge about the sea (https://www.mareano.no/kart-og-data/kartkatalog-1/ngu-kartkatalog).
Video files (with metadata/navigation data) are shared upon request within one month after the cruise. These data are shared via a file-sharing solution upon request to Kjell Bakkeplass (kjell.bakkeplass@hi.no) or Pål Buhl-Mortensen (paal.buhl.mortensen@hi.no).
Seabed observations from the field (georeferenced observation logs for geology and biology) are shared within one month after the cruise upon request to Kjell Bakkeplass (kjell.bakkeplass@hi.no) or Pål Buhl-Mortensen (paal.buhl.mortensen@hi.no).
11 - References
Brenke, N. (2005). An epibenthic sledge for operations on marine soft bottom and bedrock. Marine Technology Society Journal, 39(2), 10-21.
Burgos, J. M., Buhl-Mortensen, L., Buhl-Mortensen, P., Ólafsdóttir, S. H., Steingrund, P., Ragnarsson, S., and Skagseth, Ø. (2020). Predicting the Distribution of Indicator Taxa of Vulnerable Marine Ecosystems in the Arctic and Sub-arctic Waters of the Nordic Seas. Front. Mar. Sci. 7, 1–25. doi:10.3389/fmars.2020.00131.
Hopkins, T. S. (1991). The GIN Sea-A synthesis of its physical oceanography and literature review 1972-1985. Earth Sci. Rev. 30, 175–318. doi:10.1016/0012-8252(91)90001-V.
Meyer, H.K., Davies, A.J., Roberts, E.M., Xavier, J.R., Ribeiro, P.A., Glenner, H., Birkely, S.-R., Rapp, H.T. (2023). Beyond the tip of the seamount: Distinct megabenthic communities found beyond the charismatic summit sponge ground on an Arctic seamount (Schulz Bank, Arctic Mid-Ocean ridge). Deep Sea Research Part I: Oceanographic Research Papers, 191: 103920. DOI: http://dx.doi.org/10.1016/j.dsr.2022.103920.
Pedersen, R. B., Olsen, B. R., Barreyre, T., Bjerga, A., Denny, A., Heggernes Eilertsen, M., Fer, I., Haflidason, H., Hestetun, J. T., Jørgensen, S., Ribeiro, P. A., Steen, I. H., Stubseid, H., Tandberg, A. H. S., and Thorseth, I. (2021). Fagutredning mineralressurse i Norskehavet landskapstrekk, naturtyper og benthiske økosystemer. Bergen.
Roberts, E.M., Mienis, F., Rapp, H.T., Hanz, U., Meyer, H.K., Davies, A.J. (2018). Oceanographic setting and short-timescale environmental variability at an Arctic seamount sponge ground. Deep Sea Research Part I: Oceanographic Research Papers, 138, 98–113. https://doi.org/10.1016/j.dsr.2018.06.007.
Roberts, E. M., Bowers, D. G., Meyer, H. K., Samuelsen, A., Rapp, H. T., and Cárdenas, P. (2021). Water masses constrain the distribution of deep-sea sponges in the North Atlantic Ocean and Nordic Seas. Marine Ecology Progress Series, 659, 75–96. doi:10.3354/meps13570.
Ross, R., Thorsnes, T., Meyer, H.K., Bjarnadóttir, L., Molina, È.J., Dolan, M., Hodnesdal, H., Welde, H., Birkely, S-R., Boitsov, S., Buhl-Mortensen, P., Chand, S., Gonzalez-Mirelis, G., Holte, B., Jensen, G., Jensen, H., Johansen, Y., Kartveit, K.H., Malmquist, C., Marquez, J.F., Meek, T.J., Moy, F., Ofstad, A.E., Olssøn, R., Piechaud, N., Plotkin, A., Schimel, A., Voronkov, A., Westgaard, J.-I.(2025). MAREANO’s Deep-sea survey strategy. V4, 66 pp. Unpublished.
Santos, M. F. L. D., Pires-Vanin, A. M. S., & Muniz, P. (1996). A simple and efficient device for sorting large marine benthic samples. Revista Brasileira de Oceanografia, 44, 57-60.
12 - Appendix
Appendix 1. Overview of samples collected at the full stations (excluding the box corer samples, see Appendix 2) extracted from Marbunn.
| Station | Date | Sample no | Depth (m) | Comment |
| Trawl | ||||
| 3753 | 03.10.25 | 1 | 2678 | Agassiz trawl. 1 cm mesh size on inner net. TRANSPONDER POSTION and DEPTH. Very small sample. Sample was sorted on board. Voucher: Kolga sp. (1/6): 136 stk, 0.05 kg, EtOH,ss=100). Hymnaster (2/6): 2 stk, 0.001 kg, EtOH,ss=100). Crustacea (3/6): EtOH,ss=100). Porifera (4/6): 11 stk, 0.013 kg, EtOH,ss=100). Jellyfish (5/6): 2 stk, 0.006 kg, EtOH,ss=100). Animalia indet (6/6): 2 stk, 0.003 kg, EtOH,ss=100). SL: RO. Subsample: 100 percent. 10_mm: 5x0.5L, 1x0.3L (Kolga sp.) Photo: 234-237. |
| 3753 | 07.10.25 | 2 | 2617 | Beam trawl. About 2/3 tub of mud, some stones and gravel. Used the normal beam trawl with 5_mm inner mesh. Trawl catch sieved over a 2_mm sieve. Net under chains forn. SL: RO. Subsample: 100 percent. 5_mm: 1x0.5L, 1x5L, EtOH to Tromsø¸. 0.5_mm: 1x3L, EtOH to Bergen Museum. Photo: 291-298. |
| 3763 | 11.10.25 | 3 | 2293 | Beam trawl. 1/3 black tub. Kolga dominating. Many species seen from video. 5 buckets of mixed animals. 8 containers with fragile, rare or animals on rocks. Frozen fauna: Lycodes figidus, 15 stk, 2.017 kg, ss_100, frozen for Rupert W. Paraliparis, 1 stk, 0.080 kg, ss_100, frozen for Rupert W. Pieces of wood, 10 stk, 0.0077 kg, ss_100, frozen. Subsample: 100 percent. 0.5_mm: 1x10 L (EtOH). 5_mm: 1x0.05 L,3x0.3 L,1x0.5 L,1x1 L,2x3 L, 5x10 L (13 buckets EtOH). |
| 3763 | 10.10.25 | 4 | 2276 | Agassiz trawl. The whole catch was not put through the sieving table. It was processed straight from the bucket and poured into 1_mm sieve. Under, a 0.5_mm sieve was placed to collect smaller stuff. Discarded fauna: Lycodes frigidus, 3 stk, 1.27 kg (discarded) in ss_100. Frozen fauna: Cirroteuthis muelleri, 1 stk, 0.237 kg (frozen) in ss_100. Lycodes sp., 8 stk, 0.283 kg (frozen for Rupert W.) in ss_100. |
| Gravity corer | ||||
| 3753 | 06.10.25 | 1 | 2670 | 435 cm length. NGU_nb=143035. |
| 3763 | 10.10.25 | 2 | 2274 | 5 tubes 1 bag 0-4 cm 1 bag with catches sediment. Core length 4.78 m (+ 4 cm). Top slightly cut (0.0-4 cm) -> in a bag. Core catches apr. 20 cm -> in a bag. |
| Multicorer | ||||
| 3753 | 06.10.25 | 1 | 2672 | 6 cores (NGU:4, IMR:2) |
| 3763 | 11.10.25 | 2 | 2274 | 6 cores (NGU:5, IMR:1). Hulrom ved 9.5-10 cm. Mye forams 15-18 cm, mindre forams fra 18 cm. |
| RP-sledge | ||||
| 3753 | 04.10.25 | 1 | 2678 | Very small sample. Subsample 100 percent. 0.5_EL: 1x0.1L (EtOH) 0.5_HF: 1x0.3L (EtOH) |
| 3753 | 06.10.25 | 2 | 2681 | MISS. Cod-end gone. |
| 3763 | 11.10.25 | 3 | 2265 | MISS. Net came loose. |
| 3763 | 11.10.25 | 4 | 2285 | Subsample: 100 percent. EtOH. 0.5_mm dec: 1x1 L, 2x5 L. 4_mm: 4x10 L. 1_mm: 5x10 L. |
Appendix 2. Overview of box corer samples and their quality for cruise 2025007011. Quality type indicated by color: bad (red), good (green), and questionable (orange).
| Date | R number | Sample no | Gear | Sediment Interval [cm] (OW=Overlaying Water) | Mesh size [mm] | Elutriation fraction (EL=Elutriated; HF=Heavy fraction) | Fixative | Area subsample [m^2] | Fractioning | Container size [ml] | Qualitative/ Quantitative | Comments |
| 03.10.2025 | 3753 | 1 | Small boxcore (0.1 m^2) | 0-5 | 1 | NA | EtOH | NA | Fractioned | 300 | Qualitative | Only some of the surface layer scarped from chemistry boxcore |
| 03.10.2025 | 3753 | 1 | Small boxcore (0.1 m^2) | 0-5 | 0.5 | NA | EtOH | NA | Fractioned | 300 | Qualitative | |
| 03.10.2025 | 3753 | 1 | Small boxcore (0.1 m^2) | 0-5 | 0.3 | NA | EtOH | NA | Fractioned | 300 | Qualitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 300 | Quantitative | Bad sample. From ROV video we see boxcore smashed tiwce against the seafloor before taking sample. Probably all surface layer is gone. Boxcorer released by the ROV. Forgot to take eDNA and sediment pigments. |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 04.10.2025 | 3753 | 4 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 5000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 100 | Quantitative | Cannot see the landing on the ROV video. Probably good sample. The mud cloud is not too big, suggesting controlled landing |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 500 | Quantitative | |
| 06.11.2025 | 3753 | 5 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 10000 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 100 | Quantitative | Perfect landing. Undisturbed surface. Very good sample! |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 06.11.2025 | 3753 | 6 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 10000 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 300 | Quantitative | Bad sample. Bad landing. A lot of sediment being lifted and exiting the box through the box flaps. Banged heavily against the Aframe when put on deck due to waves. Surface very disturbed. Cracked and mixed surface. Treat results with caution. |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 1000 | Quantitative | |
| 06.11.2025 | 3753 | 7 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 10000 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 300 | Quantitative | Very good boxcore. Well preserved surface. Some organisms visible in the 1 mm sieve. Also quite abundant smaller organims in the 0,5 and 0,3 fractions. Abunant Bathyarca on sruface. Perfect landing. |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 10.11.2025 | 3753 | 8 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 5000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 300 | Quantitative | Perfect landing. Well preserved surface. Some Kolga, Bathycrinus and Sabellidae tubes + Bathyarca. Some polychaetes in the 1 mm. Also abundant fauna in the 0,5 and 0,3 mm. |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 1000 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 10.11.2025 | 3753 | 9 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 10000 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 300 | Quantitative | Perfect landing. Some complications with wire when closing the spade. But sample seems intact. |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 11.11.2025 | 3763 | 10 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 10000 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | OW | 0.3 | NA | EtOH | 0.25 | 0,3 bulk | 300 | Quantitative | Good landing. However, boxcore landed too close to previous boxcore hole. Probably disturbed surface sediment! |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | EtOH | 0.125 | Fractioned | 1000 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 0-5 | 1 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 0-5 | 0.5 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 0-5 | 0.3 | NA | Formalin | 0.125 | Fractioned | 500 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | EtOH | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | EL | Formalin | 0.125 | 0,3 bulk | 300 | Quantitative | |
| 11.11.2025 | 3763 | 11 | Big boxcore (0.25 m^2) | 5-15 | 0.3 | HF | Formalin | 0.25 | 0,3 bulk | 10000 | Quantitative |
Appendix 3. Overview of biological samples with the respective event ID and sample lot numbers collected with NORMAR ROV Ægir6000 and how they were preserved in the lab. The first line is a template example of how the rest of the data is formatted.
| Cruise number: 2025007011 | ROV Samples Data Sheet | |||||||||||||||||
| * ID number is never repeated during a cruise! No 2 samples can have the same ID number, even when starting a new dive. Keep track of the last ID number from the previous dive! Samp-ID is the Sample ID, where 1 sample could be stored in multiple containers. | Gear Types: BL = blade corer (formerly BC), CL = claw, FL = Fluid Sampler, FS = frankenscoop, NE = net, NB = Niskin Bottle, PC = push corer, SC = scoop, SH = shovel, SN = Sniffer, SS = suction sampler, TP = Temperature Probe | Storage Types: BB-ID = biobox 1,2,3,4, BL-ID = blade corer 1,2,3,4 (formerly BC), D = drawer, DL= drawer left, DR = drawer right, SS-ID = Suction sampler chamber A,B,C,D,… (SS-0 for hose), TB-ID = toolbox 1,2, TMS = above on the TMS | ||||||||||||||||
| Event ID | Samp Lot | R station | VL number | Gear | Storage | Date | SFO Time stamp | Lat (DD) SFO | Long (DD) SFO | Depth (m) SFO | Video Sample Name | Physical Sample Name | Vial size (ml) | Picked/ Bulk | Blade corer subsample ship (%) | Fix | Comments | |
| #### | #### | R#### | VL#### | Gear Type | Storage ID | dd.mm.yyyy | HH:MM:SS | ##.#### | ##.#### | #### | Name in SFO | Name of Physical Sample | ### ML | Type | Anything else needed to say | |||
| 1 | 1 | 3740 | 3831 | FS | DL-TB1 | 26.09.2025 | 14:51:22 | 72.5627 | 1.7379 | 1649.6 | Spinularia njordi + Ophiuroidea | Spinularia njordi inc. | 300 | Picked | EtOH | |||
| 1 | 2 | 3740 | 3831 | FS | DL-TB1 | 26.09.2025 | 14:51:22 | 72.5627 | 1.7379 | 1649.6 | Spinularia njordi + Ophiuroidea | Porifera indet. | 100 | Picked | EtOH | |||
| 1 | 3 | 3740 | 3831 | FS | DL-TB1 | 26.09.2025 | 14:51:22 | 72.5627 | 1.7379 | 1649.6 | Spinularia njordi + Ophiuroidea | Ophiocten sericeum | 100 | Picked | EtOH | |||
| 1 | 4 | 3740 | 3831 | FS | DL-TB1 | 26.09.2025 | 14:51:22 | 72.5627 | 1.7379 | 1649.6 | Spinularia njordi + Ophiuroidea | Sediment bulk | 1000 | Bulk | EtOH | |||
| 2 | 1 | 3740 | 3831 | NE | DR-TB2 | 26.09.2025 | 16:13:11 | 72.5590 | 1.7366 | 1667.9 | Cerianthidae | Cerianthidae | 50 | Picked | EtOH | Likely not Cerianthid, in image catalogue - Anemone dark purple | ||
| 2 | 2 | 3740 | 3831 | NE | DR-TB2 | 26.09.2025 | 16:13:11 | 72.5590 | 1.7366 | 1667.9 | Cerianthidae | Sediment bulk | 1000 | Bulk | EtOH | |||
| 3 | 3740 | 3831 | D | 26.09.2025 | Sediment discarded, no sample | |||||||||||||
| 4 | 1 | 3741 | 3832 | SS | SSA | 28.09.2025 | 08:07:46 | 72.5513 | 1.5496 | 1765.5 | Bythocaris + Geodia | Geodia parva | 3000 | Picked | EtOH | Bithocaris missing | ||
| 5 | 1 | 3741 | 3832 | NE | DL-BB | 28.09.2025 | 08:20:31 | 72.5513 | 1.5496 | 1765.5 | Porifera | Tentorium semisuberites | 100 | Picked | EtOH | Tentorium on a rock, rock given to geologists | ||
| 5 | 2 | 3741 | 3832 | NE | DL-BB | 28.09.2025 | 08:20:31 | 72.5513 | 1.5496 | 1765.5 | Porifera | Bulk sediment | 1000 | Bulk | EtOH | Leftover sediment | ||
| 6 | 1 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Bithocaris | 50 | Picked | EtOH | Only Bithocaris | ||
| 6 | 2 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Geodia | 3000 | Picked | EtOH | Geodia suctioned up when collecting nudibranch | ||
| 6 | 3 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Craniella sp. | 50 | Picked | EtOH | Small craniella sucked in when sampling | ||
| 6 | 4 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Branching hexactinellida | 100 | Picked | EtOH | Branching hexact on geodia | ||
| 6 | 5 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Amphidiscella monai | 300 | Picked | EtOH | On geodia | ||
| 6 | 6 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Nudibranchia | 50 | Picked | EtOH | Small pink nudibranch | ||
| 6 | 7 | 3741 | 3832 | SS | SSB | 28.09.2025 | 09:17:55 | 72.5526 | 1.5456 | 1671.5 | Nudibranchia + Bythocaris | Sediment bulk | 500 | Bulk | EtOH | Leftover sediment | ||
| 7 | 1 | 3741 | 3832 | SS | SSC | 28.09.2025 | 09:20:15 | 72.5526 | 1.5456 | 1671.5 | Craniella | Craniella sp. | 1000 | Picked | EtOH | Craniella | ||
| 7 | 2 | 3741 | 3832 | SS | SSC | 28.09.2025 | 09:20:15 | 72.5526 | 1.5456 | 1671.5 | Craniella | Sediment bulk | 300 | Bulk | EtOH | Leftover sediment | ||
| 8 | 1 | 3741 | 3832 | CL | DR | 28.09.2025 | 11:00:58 | 72.5553 | 1.5373 | 1632.0 | Porifera branching (not Lissodendoryx) | Sediment bulk | 500 | Bulk | EtOH | Spicules with sediments | ||
| 8 | 2 | 3741 | 3832 | CL | DR | 28.09.2025 | 11:00:58 | 72.5553 | 1.5373 | 1632.0 | Porifera branching (not Lissodendoryx) | Geodia parva | 1000 | Picked | EtOH | Next to Stelletta raphidiophora in video | ||
| 8 | 3 | 3741 | 3832 | CL | DR | 28.09.2025 | 11:00:58 | 72.5553 | 1.5373 | 1632.0 | Porifera branching (not Lissodendoryx) | Stelletta rhaphidiophora | 1000 | Picked | EtOH | Next to Geodia paro in video | ||
| 8 | 4 | 3741 | 3832 | CL | DR | 28.09.2025 | 11:00:58 | 72.5553 | 1.5373 | 1632.0 | Porifera branching (not Lissodendoryx) | Porifera branching | 1000 | Picked | EtOH | Or Geodia parva | ||
| 9 | 1 | 3741 | 3832 | D | 28.09.2025 | Sediment bulk drawer | 100 | Bulk | EtoOK | Leftover in drawer | ||||||||
| 9 | 2 | 3741 | 3832 | D | 28.09.2025 | Polychaeta Syllidae | 100 | Picked | EtOH | Leftover in drawer | ||||||||
| 10 | 3741 | 3832 | SS | SS0 | 28.09.2025 | Leftover suction sample | 50 | Bulk | EtOH | Leftover in space suctionsampler chamber | ||||||||
| 11 | 1 | 3742 | 3833 | CL | DL-BB | 28.09.2025 | 16:20:49 | 72.5330 | 1.4829 | 1297.0 | Skeleton coral | Cf Coral skeleton | 50 | Picked | EtOH | |||
| 14 | 1 | 3742 | 3833 | D | 28.09.2025 | 16:18:35 | Bulk | Bulk | 1000 | Bulk | EtOH | |||||||
| 15 | 1 | 3743 | 3834 | PC | PC-O | 28.09.2025 | 23:06:04 | 72.5372 | 1.5364 | 1560.7 | Actiniaria epibiont | Actiniaria epibiont | 300 | Picked | EtOH | On sabellidae tube | ||
| 16 | 1 | 3744 | 3835 | CL | DR | 29.09.2025 | 06:45:41 | 72.4910 | 1.9772 | 2163.7 | Rock with Ascidiacea + Porifera | Porifera encrusting green/yellow | 50 | Picked | EtOH | Rock for geology Picture taken from rock for some of the encrusting sponges (not possible to scrape off) | ||
| 16 | 2 | 3744 | 3835 | CL | DR | 29.09.2025 | 06:45:41 | 72.4910 | 1.9772 | 2163.7 | Rock with Ascidiacea + Porifera | Ascidiacea encrusting colonial and varia | 50 | Picked | EtOH | |||
| 16 | 3 | 3744 | 3835 | CL | DR | 29.09.2025 | 06:45:41 | 72.4910 | 1.9772 | 2163.7 | Rock with Ascidiacea + Porifera | Bulk | 50 | Bulk | EtOH | Debries from rock | ||
| 17 | 1 | 3744 | 3835 | CL | DR | 29.09.2025 | 06:50:10 | 72.4910 | 1.9773 | 2163.6 | Small rock with Pectinidae attached | Pectinidae | 50 | Picked | EtOH | Rock for geology | ||
| 18 | 1 | 3744 | 3835 | CL | DL | 29.09.2025 | 08:58:24 | 72.4941 | 1.9624 | 1947.4 | Rock for geologists with encrusting sponge | Porifera encrusting | 50 | Picked | EtOH | Rock for geology | ||
| 18 | 2 | 3744 | 3835 | CL | DL | 29.09.2025 | 08:58:24 | 72.4941 | 1.9624 | 1947.4 | Rock for geologists with encrusting sponge | Encrusting fauna varia | 50 | Picked | EtOH | |||
| 18 | 3 | 3744 | 3835 | CL | DL | 29.09.2025 | 08:58:24 | 72.4941 | 1.9624 | 1947.4 | Rock for geologists with encrusting sponge | Ascidiacea encrusting colonial | 50 | Picked | EtOH | |||
| 19 | 1 | 3744 | 3835 | CL | DL | 29.09.2025 | 08:59:25 | 72.4941 | 1.9623 | 1947.5 | Rock for geologists with crinoidea | Crinoidea | 50 | Picked | EtOH | Rock for geology | ||
| 19 | 2 | 3744 | 3835 | CL | DL | 29.09.2025 | 08:59:25 | 72.4941 | 1.9623 | 1947.5 | Rock for geologists with crinoidea | Porifera stalked | 50 | Picked | EtOH | Scraped from rock | ||
| 23 | 1 | 3744 | 3835 | D | 29.09.2025 | Hexactinellidae | 100 | Picked | EtOH | ID 23 is leftovers from drawer from R3744, VL3835+VL3836. | ||||||||
| 23 | 2 | 3744 | 3835 | D | 29.09.2025 | Isopoda spp. | 100 | Picked | EtOH | ID 23 is leftovers from drawer from R3744, VL3835+VL3836. | ||||||||
| 23 | 3 | 3744 | 3835 | D | 29.09.2025 | Porifera | 100 | Picked | EtOH | ID 23 is leftovers from drawer from R3744, VL3835+VL3836. | ||||||||
| 23 | 4 | 3744 | 3835 | D | 29.09.2025 | Bulk drawer sediment | 500 | Bulk | EtOH | ID 23 is leftovers from drawer from R3744, VL3835+VL3836. | ||||||||
| 20 | 1 | 3744 | 3836 | SS | SS-D | 29.09.2025 | 11:23:52 | 72.4967 | 1.9491 | 1915.3 | Asteroidea | Asteroidea | 50 | Picked | EtOH | plus some debrie fixed together with Asteroidea | ||
| 21 | 1 | 3744 | 3836 | SS | SS-E | 29.09.2025 | 11:26:43 | 72.4968 | 1.9500 | 1916.1 | Antedonoidea | Antedonoidea | 100 | Picked | EtOH | Most individuals fragmented by SS. Discs intact. | ||
| 21 | 2 | 3744 | 3836 | SS | SS-E | 29.09.2025 | 11:26:43 | 72.4968 | 1.9500 | 1916.1 | Antedonoidea | Bulk | 300 | Bulk | EtOH | Debries + fragments of Antedonoidea | ||
| 22 | 1 | 3744 | 3836 | SS | SS0 | 29.09.2025 | SS leftovers | 100 | Bulk | EtOH | ||||||||
| NA | 1 | 3744 | 3836 | CL | Front drawer | 29.09.2025 | Porifera | 500 | Picked | EtOH | One porifera from this dive preserved from stone collected without event ID#. Stored in front drawer. | |||||||
| 24 | 1 | 3745 | 3837 | NE | TB-2 | 29.09.2025 | 18:12:45 | 72.4754 | 1.9932 | 2651.8 | Elpidia sp. | Elpidia sp. | 100 | Picked | EtOH | |||
| 24 | 2 | 3745 | 3837 | NE | TB-2 | 29.09.2025 | 18:12:45 | 72.4754 | 1.9932 | 2651.8 | Elpidia sp. | Bulk | 100 | Bulk | EtOH | |||
| 25 | 1 | 3745 | 3837 | CL | DL | 29.09.2025 | 18:49:54 | 72.4761 | 1.9982 | 2580.2 | Rock with sponges | Cf. Lissodendoryx | 300 | Pick | EtOH | |||
| 25 | 2 | 3745 | 3837 | CL | DL | 29.09.2025 | 18:49:54 | 72.4761 | 1.9982 | 2580.2 | Rock with sponges | Ascidiacea encrusting | 50 | Pick | EtOH | cf Didemnidae | ||
| 25 | 3 | 3745 | 3837 | CL | DL | 29.09.2025 | 18:49:54 | 72.4761 | 1.9982 | 2580.2 | Rock with sponges | Porifera encrusting | 50 | Pick | EtOH | |||
| 26 | 1 | 3746 | 3838 | SS | SS-A | 29.09.2025 | 22:04:55 | 72.4835 | 2.0373 | 2414.5 | Bathycrinus sp. | Bathycrinus sp | 50 | Picked | EtOH | |||
| 26 | 2 | 3746 | 3838 | SS | SS-A | 29.09.2025 | 22:04:55 | 72.4835 | 2.0373 | 2414.5 | Bathycrinus sp. | Sediment bulk | 100 | Bulk | EtOH | |||
| 26 | 3 | 3746 | 3838 | SS | SS-A | 29.09.2025 | 22:04:55 | 72.4835 | 2.0373 | 2414.5 | Bathycrinus sp. | Gersemia sp. | 50 | Picked | EtOH | |||
| 27 | 1 | 3746 | 3838 | CL | DR-TB1 | 29.09.2025 | 22:16:39 | 72.4834 | 2.0372 | 2414.9 | Gersemia sp. | Gersemia sp. | 100 | Picked | EtOH | |||
| 28 | 1 | 3746 | 3838 | NE | DR | 29.09.2025 | 22:47:21 | 72.4836 | 2.0316 | 2380.0 | Irregular equinoderms (Pourtalesia sp.) | Pourtalesia fragment | 50 | Picked | EtOH | |||
| 28 | 2 | 3746 | 3838 | NE | DR | 29.09.2025 | 22:47:21 | 72.4836 | 2.0316 | 2380.0 | Irregular equinoderms (Pourtalesia sp.) | Bulk | 300 | Bulk | EtOH | |||
| 29 | 1 | 3746 | 3838 | D | 29.09.2025 | Bulk | 300 | Bulk | EtOH | Bulk sample from Drawer: clustered dive R3745 and R3746 | ||||||||
| 30 | 1 | 3747 | 3839 | CL | DL-TB2 | 30.09.2025 | 05:12:59 | 72.5413 | 2.1467 | 971.6 | Bamboo coral | Bamboo coral Keratoisidae | 5000 | Picked | EtOH | |||
| 31 | 1 | 3747 | 3839 | NE | DL | 30.09.2025 | 05:17:04 | 72.5413 | 2.1466 | 971.7 | Ophiocten sp. | Ophiocten sp. | 100 | Picked | EtOH | All sediment given to geology (bioclastic sediment) | ||
| 31 | 2 | 3747 | 3839 | NE | DL | 30.09.2025 | 05:17:04 | 72.5413 | 2.1466 | 971.7 | Ophiocten sp. | Polychaeta indet. | 50 | Picked | EtOH | (Sabellidae) taken out of tube, preserved with tube | ||
| 32 | 1 | 3747 | 3839 | FS | TDR-TB1 | 30.09.2025 | 06:32:31 | 72.5439 | 2.1540 | 956.0 | Scoop coral rubble for associated fauna | Cyclopecten sp. | 100 | Picked | EtOH | |||
| 32 | 2 | 3747 | 3839 | FS | TDR-TB1 | 30.09.2025 | 06:32:31 | 72.5439 | 2.1540 | 956.0 | Scoop coral rubble for associated fauna | Polynoidea sp. | 100 | Picked | EtOH | |||
| 32 | 3 | 3747 | 3839 | FS | TDR-TB1 | 30.09.2025 | 06:32:31 | 72.5439 | 2.1540 | 956.0 | Scoop coral rubble for associated fauna | Bulk (coral rubble and fauna) | 5000 | Bulk | EtOH | |||
| 33 | 1 | 3747 | 3839 | D | 30.09.2025 | Bulk from Drawer | 100 | Bulk | EtOH | |||||||||
| 33 | 2 | 3747 | 3839 | D | 30.09.2025 | Amphipoda indet. | 50 | Picked | EtOH | Comes from left overs drawer | ||||||||
| 35 | 1 | 3748 | 3840 | BL2 | 30.09.2025 | 14:37:37 | 72.4342 | 2.1779 | 3237.5 | Blade corer for biology | Blade corer Bulk 1mm | 300 | Bulk | 100 | EtOH | |||
| 35 | 2 | 3748 | 3840 | BL2 | 30.09.2025 | 14:37:37 | 72.4342 | 2.1779 | 3237.5 | Blade corer for biology | Blade corer Bulk 0.5 mm | 100 | Bulk | 100 | EtOH | |||
| 35 | 3 | 3748 | 3840 | BL2 | 30.09.2025 | 14:37:37 | 72.4342 | 2.1779 | 3237.5 | Blade corer for biology | Blade corer Bulk 0.3 | 50 | Bulk | 100 | EtOH | |||
| 37 | 1 | 3748 | 3840 | PC | PC-A | 30.09.2025 | 18:56:57 | 72.4313 | 2.1977 | 3231.4 | Push corer for geology | Polychaeta indet. | 50 | Picked | EtOH | From geo sample | ||
| 38 | 1 | 3749 | 3841 | NE | TB-1 | 30.09.2025 | 23:33:16 | 72.4318 | 2.0483 | 3091.5 | Saduria sp. | Saduria sp. | 100 | Picked | EtOH | |||
| 38 | 2 | 3749 | 3841 | NE | TB-1 | 30.09.2025 | 23:33:16 | 72.4318 | 2.0483 | 3091.5 | Saduria sp. | Bulk sediment | 100 | Bulk | EtOH | |||
| 40 | 1 | 3749 | 3841 | NE | DR-TB2 | 01.10.2025 | 00:46:33 | 72.4331 | 2.0593 | 3054.9 | Thenea-like | Porifera cf. Thenea | 50 | Picked | EtOH | |||
| 40 | 2 | 3749 | 3841 | NE | DR-TB2 | 01.10.2025 | 00:46:33 | 72.4331 | 2.0593 | 3054.9 | Thenea-like | Bulk | 100 | Bulk | EtOH | |||
| 41 | 1 | 3749 | 3841 | PC | PC-B | 01.10.2025 | 02:06:57 | 72.4343 | 2.0703 | 3068.6 | Pushcore with Lissodendoryx | Lissodendoryx | 300 | Picked | EtOH | Picked from geology push core | ||
| 42 | 1 | 3749 | 3841 | PC | PC-I | 01.10.2025 | 03:44:13 | 72.4348 | 2.0404 | 3198.1 | Pushcore for geologists | Polychaeta tubes | 50 | Picked | EtOH | Picked from geology push core (two different polychaeta tubes) | ||
| 43 | 1 | 3750 | 3842 | NE | DL-BB | 01.10.2025 | 04:51:03 | 72.4363 | 2.0447 | 3200.7 | Pectinidae, Pycnogonida, Bathyphelia, Fluff patch | Pourtalesia sp. | 50 | Picked | EtOH | Pourtalesia cf. Jeffreysii (with small bivalvia attached) | ||
| 43 | 2 | 3750 | 3842 | NE | DL-BB | 01.10.2025 | 04:51:03 | 72.4363 | 2.0447 | 3200.7 | Pectinidae, Pycnogonida, Bathyphelia, Fluff patch | Pycnogonida indet. | 50 | Picked | EtOH | Ascorhynchus abyssi (cf.) | ||
| 43 | 3 | 3750 | 3842 | NE | DL-BB | 01.10.2025 | 04:51:03 | 72.4363 | 2.0447 | 3200.7 | Pectinidae, Pycnogonida, Bathyphelia, Fluff patch | Bathyphellia sp. | 50 | Picked | EtOH | |||
| 43 | 4 | 3750 | 3842 | NE | DL-BB | 01.10.2025 | 04:51:03 | 72.4363 | 2.0447 | 3200.7 | Pectinidae, Pycnogonida, Bathyphelia, Fluff patch | Bulk sediment | 1000 | Bulk | EtOH | |||
| 44 | 1 | 3750 | 3842 | PC | PC-H | 01.10.2025 | 06:28:14 | 72.4402 | 2.0561 | 3200.2 | Geo sampling with Sabellidae tube | Polychaeta tube | 50 | Picked | EtOH | Picked from geology push core | ||
| 45 | 1 | 3749/3750 | 3841/3842 | D | 01.10.2025 | Drawer bulk sediment | 100 | Bulk | EtOH | Drawer left over from cluster dive R3749-R3750 and VL3841-VL3842 | ||||||||
| 49 | 1 | 3753 | 3844 | NE | DL-TB1 | 03.10.2025 | 12:07:06 | 72.2970 | 1.8863 | 2680.5 | Biology debries or sponge + sea urchin shells | Bulk bio debries | 300 | Bulk | EtOH | |||
| 49 | 2 | 3753 | 3844 | NE | DL-TB1 | 03.10.2025 | 12:07:06 | 72.2970 | 1.8863 | 2680.5 | Biology debries or sponge + sea urchin shells | Kolga sp. | 50 | Picked | EtOH | Picture 5 min after ethanol fixation | ||
| 50 | 1 | 3753 | 3844 | NE | DR-TB2 | 03.10.2025 | 12:50:50 | 72.2972 | 1.8810 | 2675.8 | Tunicate? | Candelabrum sp. | 300 | Picked | EtOH | Picture 5 min after ethanol fixation | ||
| 50 | 2 | 3753 | 3844 | NE | DR-TB2 | 03.10.2025 | 12:50:50 | 72.2972 | 1.8810 | 2675.8 | Tunicate? | Bulk | 300 | Bulk | EtOH | |||
| 51 | 1 | 3753 | 3844 | FS | DL-BB | 03.10.2025 | 13:00:05 | 72.2972 | 1.8810 | 2675.7 | Salp? | Candelabrum sp. | 50 | Picked | EtOH | Picture 5 min after ethanol fixation | ||
| 51 | 2 | 3753 | 3844 | FS | DL-BB | 03.10.2025 | 13:00:05 | 72.2972 | 1.8810 | 2675.7 | Salp? | Sabellidae sp. | 50 | Picked | EtOH | Plus other polychaeta tubes, can be empty | ||
| 51 | 3 | 3753 | 3844 | FS | DL-BB | 03.10.2025 | 13:00:05 | 72.2972 | 1.8810 | 2675.7 | Salp? | Bulk | 300 | Bulk | EtOH | |||
| 64 | 1 | 3753 | 3844 | D | 03.10.2025 | Lysianassidae | 50 | Picked | EtOH | |||||||||
| 64 | 2 | 3753 | 3844 | D | 03.10.2025 | Kolga sp. | 50 | Picked | EtOH | |||||||||
| 64 | 3 | 3753 | 3844 | D | 03.10.2025 | Bulk | 300 | Bulk | EtOH | |||||||||
| 67 | 1 | 3754 | 3845 | BL-2 | 04.10.2025 | 12:54:45 | 72.3361 | 1.8690 | 2606.3 | Blade core for biology | Bulk BL 1 mm | 500 | Bulk | 100 | EtOH | |||
| 67 | 2 | 3754 | 3845 | BL-2 | 04.10.2025 | 12:54:45 | 72.3361 | 1.8690 | 2606.3 | Blade core for biology | Bulk BL 0.5 mm | 500 | Bulk | 100 | EtOH | |||
| 67 | 3 | 3754 | 3845 | BL-2 | 04.10.2025 | 12:54:45 | 72.3361 | 1.8690 | 2606.3 | Blade core for biology | Bulk BL 0.3 mm | 500 | Bulk | 100 | EtOH | |||
| 69 | 1 | 3754 | 3845 | BL-1 | 04.10.2025 | 14:37:53 | 72.3333 | 1.8759 | 2458.6 | Blade core for biology | Bulk BL 1 mm | 500 | Bulk | 100 | EtOH | |||
| 69 | 2 | 3754 | 3845 | BL-1 | 04.10.2025 | 14:37:53 | 72.3333 | 1.8759 | 2458.6 | Blade core for biology | Bulk BL 0.5 mm | 500 | Bulk | 100 | EtOH | |||
| 69 | 3 | 3754 | 3845 | BL-1 | 04.10.2025 | 14:37:53 | 72.3333 | 1.8759 | 2458.6 | Blade core for biology | Bulk BL 0.3 mm | 500 | Bulk | 100 | EtOH | |||
| 72 | 1 | 3752 | 3846 | NE | TB-1 | 04.10.2025 | 20:27:59 | 72.3682 | 1.9407 | 2856.8 | Gastropoda | Gastropoda | 50 | Picked | EtOH | |||
| 72 | 2 | 3752 | 3846 | NE | TB-1 | 04.10.2025 | 20:27:59 | 72.3682 | 1.9407 | 2856.8 | Gastropoda | Bulk | 300 | Bulk | EtOH | |||
| 73 | 1 | 3752 | 3846 | PC | PC-H | 04.10.2025 | 20:59:50 | 72.3680 | 1.9468 | 2831.9 | Gravel push core | Cladoritzidae | 50 | Picked | EtOH | Picked from geology pushcore? | ||
| 73 | 2 | 3752 | 3846 | PC | PC-H | 04.10.2025 | 20:59:50 | 72.3680 | 1.9468 | 2831.9 | Gravel push core | Polychaeta indet. | 50 | Picked | EtOH | Picked from geology pushcore? | ||
| 75 | 1 | 3752 | 3846 | D | 04.10.2025 | Bulk from drawer | 300 | Bulk | EtOH | |||||||||
| 77 | 1 | 3755 | 3847 | NE | DL-TB1 | 05.10.2025 | 02:45:13 | 72.3301 | 1.9465 | 2631.6 | Thenea + Bathyarca + Pycnogonida and Kolga | Kolga sp. | 50 | Picked | EtOH | |||
| 77 | 2 | 3755 | 3847 | NE | DL-TB1 | 05.10.2025 | 02:45:13 | 72.3301 | 1.9465 | 2631.6 | Thenea + Bathyarca + Pycnogonida and Kolga | Thenea sp. | 100 | Picked | EtOH | |||
| 77 | 3 | 3755 | 3847 | NE | DL-TB1 | 05.10.2025 | 02:45:13 | 72.3301 | 1.9465 | 2631.6 | Thenea + Bathyarca + Pycnogonida and Kolga | Bathyarca sp. | 50 | Picked | EtOH | |||
| 77 | 4 | 3755 | 3847 | NE | DL-TB1 | 05.10.2025 | 02:45:13 | 72.3301 | 1.9465 | 2631.6 | Thenea + Bathyarca + Pycnogonida and Kolga | Bulk | 300 | Bulk | EtOH | |||
| 79 | 1 | 3756 | 3848 | BL-1 | 05.10.2025 | 12:05:55 | 72.3288 | 1.5171 | 2313.4 | Blade core on worm fields near bacterial mats | Bulk sediment on worm fields | 3000 | Bulk | 100 | EtOH | Samples for Mari Eilertsen (sieved at 300 microns) | ||
| 80 | 1 | 3756 | 3848 | BL-2 | 05.10.2025 | 12:29:40 | 72.3289 | 1.5175 | 2314.4 | Blade core on worms and bacteria | Bulk sediment on worm fields + bacteria | 1000 | Bulk | 100 | EtOH | Samples for Mari Eilertsen (sieved at 300 microns) | ||
| 88 | 1 | 3757 | 3849 | FS | DR-TB2 | 05.10.2025 | 22:56:17 | 72.3289 | 1.5190 | 2294.5 | Sediments by hydrothermal vent | Bulk sediment within 1 m away from hydrothermal vent | 10000 | Bulk | EtOH | Handle samples with care (use PPE). Unsure if it is safe to work with samples. | ||
| 93 | 1 | 3757 | 3849 | FS | DL-TB1 | 05.10.2025 | 23:31:31 | 72.3288 | 1.5188 | 2296.4 | Sediments few m away from vent | Bulk sediment few meters away from hydrothermal vent | 10000 | Bulk | EtOH | Handle samples with care (use PPE). Unsure if it is safe to work with samples. | ||
| 95 | 1 | 3756/3757 | 3848/3849 | D | 06.10.2025 | Drawer leftover from two VLs | 1000 | Bulk | EtOH | Bulk sediment from both R3756/3757 and VL3848/3849 | ||||||||
| 96 | 1 | 3759 | 3851 | NE | DL-TB1 | 09.10.2025 | 00:41:45 | 72.2144 | 1.6388 | 2429.1 | Sabellida + Bathycrinus + unkown creature on bathycrinus | Bathycrinus sp. | 200 | Picked | EtOH | |||
| 96 | 2 | 3759 | 3851 | NE | DL-TB1 | 09.10.2025 | 00:41:45 | 72.2144 | 1.6388 | 2429.1 | Sabellida + Bathycrinus + unkown creature on bathycrinus | Sabellidae sp. | 200 | Picked | EtOH | |||
| 96 | 3 | 3759 | 3851 | NE | DL-TB1 | 09.10.2025 | 00:41:45 | 72.2144 | 1.6388 | 2429.1 | Sabellida + Bathycrinus + unkown creature on bathycrinus | Actiniaria epizoic | 200 | Picked | EtOH | |||
| 97 | 1 | 3759 | 3851 | NE | DL-TB1 | 09.10.2025 | 00:49:04 | 72.2144 | 1.6387 | 2429.2 | Polymastiidae | Polymastiidae | 200 | Picked | EtOH | |||
| 97 | 1 | 3759 | 3851 | NE | DL-TB1 | 09.10.2025 | 00:49:04 | 72.2144 | 1.6387 | 2429.2 | Polymastiidae | Bulk from Net | 300 | Bulk | EtOH | Bulk from both events 96 and 97. Used net twice in the same location and put in the same tool box. | ||
| 98 | 1 | 3759 | 3851 | NE | DR-TB2 | 09.10.2025 | 01:26:22 | 72.2159 | 1.6357 | 2432.0 | Antedonoidea | Antedonoidea indet. | 300 | Picked | EtOH | |||
| 98 | 2 | 3759 | 3851 | NE | DR-TB2 | 09.10.2025 | 01:26:22 | 72.2159 | 1.6357 | 2432.0 | Antedonoidea | Sabellidae indet. | 300 | Picked | EtOH | |||
| 98 | 3 | 3759 | 3851 | NE | DR-TB2 | 09.10.2025 | 01:26:22 | 72.2159 | 1.6357 | 2432.0 | Antedonoidea | Bulk | 500 | Bulk | EtOH | |||
| 99 | 1 | 3759 | 3851 | SS | SS-B | 09.10.2025 | 02:06:29 | 72.2174 | 1.6324 | 2431.1 | Small brown balls | Small brown balls (Porifera cf.) | 300 | Picked | EtOH | |||
| 99 | 2 | 3759 | 3851 | SS | SS-B | 09.10.2025 | 02:06:29 | 72.2174 | 1.6324 | 2431.1 | Small brown balls | Kolga sp. | 300 | Picked | EtOH | |||
| 99 | 3 | 3759 | 3851 | SS | SS-B | 09.10.2025 | 02:06:29 | 72.2174 | 1.6324 | 2431.1 | Small brown balls | Sabellidae indet. | 300 | Picked | EtOH | |||
| 99 | 4 | 3759 | 3851 | SS | SS-B | 09.10.2025 | 02:06:29 | 72.2174 | 1.6324 | 2431.1 | Small brown balls | Isopoda | 300 | Picked | EtOH | |||
| 99 | 5 | 3759 | 3851 | SS | SS-B | 09.10.2025 | 02:06:29 | 72.2174 | 1.6324 | 2431.1 | Small brown balls | Bulk | 300 | Bulk | EtOH | |||
| 101 | 1 | 3759 | 3851 | SS | SS0 | 08.10.2025 | Bulk drawer | 300 | Bulk | EtOH | ||||||||
| 102 | 1 | 3760 | 3852 | NE | DL-TB1 | 09.10.2025 | 09:39:16 | 72.2767 | 1.7024 | 2665.2 | Sipunculida | Bulk | 300 | Bulk | EtOH | Aiming for a Sipunculida, but we did not find it among the sediment | ||
| 104 | 1 | 3760 | 3852 | NE | DR-TB2 | 09.10.2025 | 11:05:56 | 72.2758 | 1.6907 | 2653.4 | Porifera round | Porifera round | 200 | Picked | EtOH | Porifera not identified on board | ||
| 104 | 2 | 3760 | 3852 | NE | DR-TB2 | 09.10.2025 | 11:05:56 | 72.2758 | 1.6907 | 2653.4 | Porifera round | Isopoda indet. | 200 | Picked | EtOH | Not targeted on video | ||
| 104 | 3 | 3760 | 3852 | NE | DR-TB2 | 09.10.2025 | 11:05:56 | 72.2758 | 1.6907 | 2653.4 | Porifera round | Bulk | 300 | Bulk | EtOH | |||
| 105 | 1 | 3760 | 3852 | FS | D | 09.10.2025 | 11:47:53 | 72.2753 | 1.6851 | 2653.0 | Prosobranchia | Prosobranchia indet. | 100 | Picked | EtOH | |||
| 105 | 2 | 3760 | 3852 | FS | D | 09.10.2025 | 11:47:53 | 72.2753 | 1.6851 | 2653.0 | Prosobranchia | Isopoda indet. | 100 | Picked | EtOH | |||
| 105 | 3 | 3760 | 3852 | FS | D | 09.10.2025 | 11:47:53 | 72.2753 | 1.6851 | 2653.0 | Prosobranchia | Bulk | 300 | Bulk | EtOH | FS was placed in drawer without any box, therefore left overs from drawer are a mix from other samples and FS | ||
| 108 | 1 | 3760 | 3852 | PC | PC-A | 09.10.2025 | 12:37:50 | 72.2749 | 1.6793 | 2645.9 | Push core for geology on top of a Pourtalesia | Pourtalesia sp. | 300 | Picked | EtOH | Given by geologists from pushcorer | ||
| 110 | 1 | 3761 | 3853 | FS | DL-TB1 | 09.10.2025 | 17:57:08 | 72.2790 | 1.5746 | 2419.3 | Scoop of flake for geology | Porifera indet. | 300 | Picked | EtOH | Encrusting rock | ||
| 110 | 2 | 3761 | 3853 | FS | DL-TB1 | 09.10.2025 | 17:57:08 | 72.2790 | 1.5746 | 2419.3 | Scoop of flake for geology | Ascidiacea colonial | 300 | Picked | EtOH | Encrusting rock | ||
| 110 | 3 | 3761 | 3853 | FS | DL-TB1 | 09.10.2025 | 17:57:08 | 72.2790 | 1.5746 | 2419.3 | Scoop of flake for geology | Actiniaria | 300 | Picked | EtOH | On a rock | ||
| 112 | 1 | 3762 | 3854 | NE | 09.10.2025 | 22:39:53 | 72.3102 | 1.6710 | 2531.4 | Bathycrinus with epibionts (Pentocriunus, serpulida, hydrozoa on stalk) | Bathycrinus sp. | 200 | Picked | EtOH | Corrected event ID from 113 to 112. 112 is the correct ID. Paper log physics fauna lab is wrong. | |||
| 112 | 2 | 3762 | 3854 | NE | 09.10.2025 | 22:39:53 | 72.3102 | 1.6710 | 2531.4 | Bathycrinus with epibionts (Pentocriunus, serpulida, hydrozoa on stalk) | Hydrozoa indet. | 200 | Picked | EtOH | Corrected event ID from 113 to 112. 112 is the correct ID. Paper log physics fauna lab is wrong. | |||
| 112 | 3 | 3762 | 3854 | NE | 09.10.2025 | 22:39:53 | 72.3102 | 1.6710 | 2531.4 | Bathycrinus with epibionts (Pentocriunus, serpulida, hydrozoa on stalk) | cf. Eggs indet. | 200 | Picked | EtOH | Corrected event ID from 113 to 112. 112 is the correct ID. Paper log physics fauna lab is wrong. | |||
| 112 | 4 | 3762 | 3854 | NE | 09.10.2025 | 22:39:53 | 72.3102 | 1.6710 | 2531.4 | Bathycrinus with epibionts (Pentocriunus, serpulida, hydrozoa on stalk) | Bulk from Net | 500 | Bulk | EtOH | Corrected event ID from 113 to 112. 112 is the correct ID. Paper log physics fauna lab is wrong. | |||
| 122 | 1 | 3764 | 3856 | NE | DL-TB2 | 12.10.2025 | 04:19:53 | 72.3686 | 1.2568 | 2361.3 | Edwardsiidae | cf. Edwardsiidae | 300 | Picked | EtOH | |||
| 122 | 2 | 3764 | 3856 | NE | DL-TB2 | 12.10.2025 | 04:19:53 | 72.3686 | 1.2568 | 2361.3 | Edwardsiidae | Bulk from Net | 500 | Bulk | EtOH | |||
| 129 | 1 | 3766 | 3858 | CL | DR-TB2 | 12.10.2025 | 17:08:55 | 72.3126 | 1.3799 | 3233.6 | Geo/Litter (black litter with Bathyphelia?) | Actiniaria indet. | 200 | Picked | EtOH | Given from geo sampling | ||
| 129 | 2 | 3766 | 3858 | CL | DR-TB2 | 12.10.2025 | 17:08:55 | 72.3126 | 1.3799 | 3233.6 | Geo/Litter (black litter with Bathyphelia?) | Polychaeta indet. | 200 | Picked | EtOH | Given from geo sampling | ||
| 133 | 1 | 3768 | 3860 | SS | SS-B&G | 13.10.2025 | 04:25:36 | 72.4360 | 1.6150 | 2919.6 | Elpidia | Elpidia sp. | 300 | Picked | EtOH | |||
| 134 | 1 | 3768 | 3860 | SS | SS-E | 13.10.2025 | 05:33:55 | 72.4394 | 1.6110 | 2803.2 | Pycnogonida | Pycnogonida indet. | 300 | Picked | EtOH | |||
| 135 | 1 | 3768 | 3860 | SS | SS0 | 13.10.2025 | Polychaeta indet. | 200 | Picked | EtOH | SS-0, forams are thrown away (very few), only polychaeta is kept | |||||||
| 141 | 1 | 3767 | 3862 | NE | DL-TB2 | 13.10.2025 | 15:55:34 | 72.3075 | 1.4274 | 2545.8 | Gersemia sp. + Bathycrinus | Gersemia sp. + Bathycrinus sp. | 500 | Picked | EtOH | Kept together in the same jar | ||
| 142 | 1 | 3767 | 3862 | CL | Front drawer | 13.10.2025 | 17:07:25 | 72.3087 | 1.4370 | 2538.1 | Rock for geology | Sediment bulk | 500 | Bulk | EtOH | This was a geosampling of a rock. Associated fauna were collected. | ||
| 142 | 2 | 3767 | 3862 | CL | Front drawer | 13.10.2025 | 17:07:25 | 72.3087 | 1.4370 | 2538.1 | Rock for geology | Elpidia sp. | 100 | Picked | EtOH | |||
| 142 | 3 | 3767 | 3862 | CL | Front drawer | 13.10.2025 | 17:07:25 | 72.3087 | 1.4370 | 2538.1 | Rock for geology | Polychaeta tubes | 100 | Picked | EtOH | |||
| 142 | 4 | 3767 | 3862 | CL | Front drawer | 13.10.2025 | 17:07:25 | 72.3087 | 1.4370 | 2538.1 | Rock for geology | Tanaidacea | 50 | Picked | EtOH | |||
| 142 | 5 | 3767 | 3862 | CL | Front drawer | 13.10.2025 | 17:07:25 | 72.3087 | 1.4370 | 2538.1 | Rock for geology | Isopoda | 50 | Picked | EtOH | |||
| 142 | 6 | 3767 | 3862 | CL | Front drawer | 13.10.2025 | 17:07:25 | 72.3087 | 1.4370 | 2538.1 | Rock for geology | Themisto sp. | 50 | Picked | EtOH | |||
| 147 | 1 | 3770 | 3863 | NE | TB2 | 14.10.2025 | 13:27:13 | 72.4320 | 1.2779 | 1884.3 | Snail (Bulbeis cf.) | Gastropoda indet. | 200 | Picked | EtOH | |||
| 147 | 2 | 3770 | 3863 | NE | TB2 | 14.10.2025 | 13:27:13 | 72.4320 | 1.2779 | 1884.3 | Snail (Bulbeis cf.) | Bulk | 500 | Picked | EtOH | |||
| 148 | 1 | 3770 | 3863 | SS | SS-B | 14.10.2025 | 14:58:15 | 72.4355 | 1.2758 | 1809.7 | Amathillopsis/ Cleippides | Amathillopsis spinigera | 200 | Picked | EtOH | Only forams as bulk. Discarded. | ||
| 152 | 1 | 3771 | 3864 | SS | SS-G,B,C | 14.10.2025 | 21:15:04 | 72.4809 | 1.2539 | 2030.2 | Neohela (sampled 4 holes) | Neohela sp. | 300 | Picked | EtOH | |||
| 152 | 2 | 3771 | 3864 | SS | SS-G,B,C | 14.10.2025 | 21:15:04 | 72.4809 | 1.2539 | 2030.2 | Neohela (sampled 4 holes) | Bulk | 500 | Bulk | EtOH | |||
| 154 | 1 | 3772 | 3865 | SS | SS-G | 15.10.2025 | 08:25:46 | 72.4880 | -0.4190 | 1264.1 | Matrix with associated fauna | Spicule mat w associated fauna | 1000 | Bulk | EtOH | |||
| 155 | 1 | 3772 | 3865 | SS | SS-B | 15.10.2025 | 08:27:29 | 72.4880 | -0.4189 | 1263.9 | Pectinidae | Pectinidae | 300 | Picked | EtOH | |||
| 155 | 2 | 3772 | 3865 | SS | SS-B | 15.10.2025 | 08:27:29 | 72.4880 | -0.4189 | 1263.9 | Pectinidae | Porifera indet. | 300 | Picked | EtOH | |||
| 155 | 3 | 3772 | 3865 | SS | SS-B | 15.10.2025 | 08:27:29 | 72.4880 | -0.4189 | 1263.9 | Pectinidae | Bulk | 300 | Bulk | EtOH | |||
| 157 | 1 | 3772 | 3865 | NE | DR-TB1 | 15.10.2025 | 09:21:14 | 72.4867 | -0.4228 | 1250.0 | Bivalvia siphons + Ophiocten + Polychaeta | Ophiocten sp. | 300 | Picked | EtOH | |||
| 157 | 2 | 3772 | 3865 | NE | DR-TB1 | 15.10.2025 | 09:21:14 | 72.4867 | -0.4228 | 1250.0 | Bivalvia siphons + Ophiocten + Polychaeta | Polynoidae | 200 | Picked | EtOH | |||
| 157 | 3 | 3772 | 3865 | NE | DR-TB1 | 15.10.2025 | 09:21:14 | 72.4867 | -0.4228 | 1250.0 | Bivalvia siphons + Ophiocten + Polychaeta | Bulk | 1000 | Bulk | EtOH | |||
| 158 | 1 | 3772 | 3865 | NE | DL-BB | 15.10.2025 | 09:35:46 | 72.4869 | -0.4205 | 1244.6 | Teredinidae + piece of wood | Teredinidae and bulk | 500 | Bulk | EtOH | |||
| 159 | 1 | 3772 | 3865 | FS | DL-TB2 | 15.10.2025 | 10:35:10 | 72.4853 | -0.4268 | 1234.3 | Siphons on spicule bottom | Bulk spicule mat | 3000 | Bulk | EtOH | |||
| 160 | 1 | 3772 | 3865 | SS | SS-C | 15.10.2025 | 11:23:02 | 72.4840 | -0.4308 | 1221.2 | Bathyarca + rock | Bathyarca sp. | 200 | Picked | EtOH | |||
| 160 | 2 | 3772 | 3865 | SS | SS-C | 15.10.2025 | 11:23:02 | 72.4840 | -0.4308 | 1221.2 | Bathyarca + rock | Prosobranchia indet. | 200 | Picked | EtOH | |||
| 160 | 3 | 3772 | 3865 | SS | SS-C | 15.10.2025 | 11:23:02 | 72.4840 | -0.4308 | 1221.2 | Bathyarca + rock | Bulk | 500 | Bulk | EtOH | |||
| 162 | 1 | 3772 | 3865 | SS | SS-D | 15.10.2025 | 12:31:34 | 72.4826 | -0.4348 | 1177.7 | 2 x Seastars | Asteroidea indet. | 500 | Picked | EtOH | |||
| 162 | 2 | 3772 | 3865 | SS | SS-D | 15.10.2025 | 12:31:34 | 72.4826 | -0.4348 | 1177.7 | 2 x Seastars | Asteroidea indet. | 300 | Picked | EtOH | |||
| 162 | 3 | 3772 | 3865 | SS | SS-D | 15.10.2025 | 12:31:34 | 72.4826 | -0.4348 | 1177.7 | 2 x Seastars | Bulk | 500 | Bulk | EtOH | |||
| 163 | 1 | 3772 | 3865 | SS | SS-E | 15.10.2025 | 12:32:06 | 72.4826 | -0.4348 | 1177.7 | 2 x shrimps | Caridea indet. | 300 | Picked | EtOH | |||
| 165 | 1 | 3772 | 3865 | SS | SS0 | 15.10.2025 | Bulk SS leftovers | 200 | Bulk | EtOH | ||||||||
| 166 | 1 | 3772 | 3865 | D | 15.10.2025 | Bulk Drawer | 300 | Bulk | EtOH | |||||||||
| 168 | 1 | 3773 | 3866 | SS | SS-B | 15.10.2025 | 16:50:17 | 72.4699 | -0.4610 | 1164.5 | Siphons | Limatula sp. | 200 | Picked | EtOH | 1164 m | ||
| 168 | 2 | 3773 | 3866 | SS | SS-B | 15.10.2025 | 16:50:17 | 72.4699 | -0.4610 | 1164.5 | Siphons | Bulk | 300 | Bulk | EtOH | 1164 m | ||
| 169 | 1 | 3773 | 3866 | NE | DL-TB2 | 15.10.2025 | 16:54:41 | 72.4699 | -0.4610 | 1164.5 | Siphons/holes | Limatula tube | 200 | Picked | EtOH | 1164 m | ||
| 169 | 2 | 3773 | 3866 | NE | DL-TB2 | 15.10.2025 | 16:54:41 | 72.4699 | -0.4610 | 1164.5 | Siphons/holes | Bryozoa indet. | 200 | Picked | EtOH | 1164 m | ||
| 169 | 3 | 3773 | 3866 | NE | DL-TB2 | 15.10.2025 | 16:54:41 | 72.4699 | -0.4610 | 1164.5 | Siphons/holes | Bulk | 500 | Bulk | EtOH | 1164 m | ||
| 170 | 1 | 3773 | 3866 | NE | DR-TB1 | 15.10.2025 | 17:11:46 | 72.4698 | -0.4606 | 1169.6 | Eggs? | Porifera indet. | 1000 | Picked | EtOH | 1169 m (not eggs) | ||
| 171 | 1 | 3773 | 3866 | SS | SS-C | 15.10.2025 | 17:53:39 | 72.4711 | -0.4651 | 1115.9 | Siphon with green content | Limatula sp. | 300 | Picked | EtOH | 1115 m + tubes, | ||
| 171 | 2 | 3773 | 3866 | SS | SS-C | 15.10.2025 | 17:53:39 | 72.4711 | -0.4651 | 1115.9 | Siphon with green content | Ophiuroidea indet. | 300 | Picked | EtOH | 1115 m | ||
| 171 | 3 | 3773 | 3866 | SS | SS-C | 15.10.2025 | 17:53:39 | 72.4711 | -0.4651 | 1115.9 | Siphon with green content | Bulk | 300 | Bulk | EtOH | 1115 m | ||
| 173 | 1 | 3773 | 3866 | FS | DL | 15.10.2025 | 18:44:36 | 72.4721 | -0.4688 | 1056.0 | Nudibranch | Nudibranchia indet. | 300 | Picked | EtOH | 1055 m | ||
| 174 | 1 | 3774 | 3867 | SS | SS-D | 15.10.2025 | 23:22:10 | 72.4511 | -0.5346 | 1149.0 | Various taxa | Bulk | 1000 | Bulk | EtOH | 1147 m. Various spp. Hydrozoa, serpulidae, amphipoda, porifera, mollusca | ||
| 176 | 1 | 3774 | 3867 | PC | PC-G | 16.10.2025 | 00:17:28 | 72.4534 | -0.5354 | 1086.0 | Push core for geology /Bivalvia/siphons ? | Limatula sp. | 300 | Picked | EtOH | 1086 m | ||
| 176 | 2 | 3774 | 3867 | PC | PC-G | 16.10.2025 | 00:17:28 | 72.4534 | -0.5354 | 1086.0 | Push core for geology /Bivalvia/siphons ? | Bulk | 300 | Bulk | EtOH | 1086 m | ||
| 177 | 1 | 3774 | 3867 | CL | 16.10.2025 | Ciona sp. | 1000 | Picked | EtOH | 1086 m (Litter in ROV drawer). Not logged in SFO as an event. Assign station/vl coordinates and metadata | ||||||||
| 177 | 2 | 3774 | 3867 | CL | 16.10.2025 | Bathyarca sp. | 300 | Picked | EtOH | 1086 m (Litter in ROV drawer). Not logged in SFO as an event. Assign station/vl coordinates and metadata | ||||||||
| 177 | 3 | 3774 | 3867 | CL | 16.10.2025 | Bryozoa indet. | 300 | Picked | EtOH | 1086 m (Litter in ROV drawer). Not logged in SFO as an event. Assign station/vl coordinates and metadata | ||||||||
| 177 | 4 | 3774 | 3867 | CL | 16.10.2025 | Bulk | 300 | Bulk | EtOH | 1086 m (Litter in ROV drawer). Not logged in SFO as an event. Assign station/vl coordinates and metadata | ||||||||
| 178 | 1 | 3773-3774 | 3866-3867 | D | 16.10.2025 | Ptychogastria sp. | 300 | Picked | EtOH | Likely from the drawer from 3774 dive | ||||||||
| 178 | 2 | 3773-3774 | 3866-3867 | D | 16.10.2025 | Bulk | 500 | Bulk | EtOH | Drawer left overs | ||||||||
| 179 | 1 | 3773-3774 | 3866-3867 | SS | SS0 | 16.10.2025 | Lysianassidae | 300 | Picked | EtOH | ||||||||
| 179 | 2 | 3773-3774 | 3866-3867 | SS | SS0 | 16.10.2025 | Bulk | 500 | Bulk | EtOH | ||||||||
| 181 | 1 | 3775 | 3868 | FS | DL-TB2 | 16.10.2025 | 06:02:44 | 72.4346 | -0.5242 | 2389.6 | Branched sponge piece? | Porifera stalk | 300 | Picked | EtOH | |||
| 181 | 2 | 3775 | 3868 | FS | DL-TB2 | 16.10.2025 | 06:02:44 | 72.4346 | -0.5242 | 2389.6 | Branched sponge piece? | Bulk | 1000 | Bulk | EtOH | |||
| 183 | 1 | 3776 | 3869 | FS | DR-TB1 | 16.10.2025 | 14:28:41 | 72.2585 | -0.7976 | 1435.8 | Geodia/Stelletta +bacterial mat + Asteroidea | Tylaster willey | 1000 | Picked | EtOH | Bucket and vials were labelled with geat NE (net) instead of Frankenscoop (FS) | ||
| 183 | 2 | 3776 | 3869 | FS | DR-TB1 | 16.10.2025 | 14:28:41 | 72.2585 | -0.7976 | 1435.8 | Geodia/Stelletta +bacterial mat + Asteroidea | Ptychogastria sp. | 200 | Picked | EtOH | |||
| 183 | 3 | 3776 | 3869 | FS | DR-TB1 | 16.10.2025 | 14:28:41 | 72.2585 | -0.7976 | 1435.8 | Geodia/Stelletta +bacterial mat + Asteroidea | Stelletta + bacteria | 20000 | Bulk | EtOH | The Stelletta + bacteria was devided into 2 buckets of 10 L each + 1 frozen bag at -80 C. All of them were labelled as sample lot 3/3 | ||
| 184 | 1 | 3776 | 3869 | NE | DL-TB2 | 16.10.2025 | 16:00:32 | 72.2602 | -0.8147 | 1337.5 | Benthic ctenophore with some forams | Bulk | 1000 | Bulk | EtOH | |||
| 189 | 1 | 3775-3776 | 3868-3869 | D | 16.10.2025 | Bulk from drawer | 1000 | Bulk | EtOH | |||||||||
| 189 | 2 | 3775-3776 | 3868-3869 | D | 16.10.2025 | Halineges sp. | 200 | Picked | EtOH | |||||||||
| 194 | 1 | 3778 | 3871 | NE | DR-TB2 | 17.10.2025 | 02:45:15 | 72.2472 | -0.7120 | 1036.2 | Bryozoa soft bush | Bryozoa indet. | 300 | Picked | EtOH | |||
| 194 | 2 | 3778 | 3871 | NE | DR-TB2 | 17.10.2025 | 02:45:15 | 72.2472 | -0.7120 | 1036.2 | Bryozoa soft bush | Bulk from th Net | 500 | Bulk | EtOH | |||
| 195 | 1 | 3778 | 3871 | D | 17.10.2025 | Bulk from drawer | 500 | Bulk | EtOH | This event was not recorded in SFO. Could be the drawer leftovers. Unsure | ||||||||
| 195 | 2 | 3778 | 3871 | D | 17.10.2025 | Ophiocten sp. | 300 | Picked | EtOH | |||||||||
| 197 | 1 | 3379 | 3872 | NE | DL-TB1 | 17.10.2025 | 06:30:41 | 72.2577 | -0.5855 | 966.2 | Capitellidae | Capitellidae | 300 | Picked | EtOH | |||
| 197 | 2 | 3379 | 3872 | NE | DL-TB1 | 17.10.2025 | 06:30:41 | 72.2577 | -0.5855 | 966.2 | Capitellidae | Bulk | 1000 | Bulk | EtOH | |||
| 198 | 1 | 3379 | 3872 | CL | DR-TB2 | 17.10.2025 | 06:36:10 | 72.2578 | -0.5855 | 966.2 | Keratoisididae | Keratoisididae | 5000 | Picked | EtOH | |||