Prosjektet hadde som mål å undersøke velferdspåvirkningen av enkelt- og kombinerte avlusingstiltak for regnbueørret, samt deres effektivitet i å fjerne lakselus. Dette inkluderte ferskvannsbad, termisk eksponeringer og deres sekvensielle bruk hos regnbueørret (Oncorhynchus mykiss) for å fylle kritiske kunnskapshull sammenlignet med atlantisk laks. Et omfattende sett med eksperimenter ble gjennomført for å fastslå artsspesifikke responser hos ørret på behandlingene, inkludert forsøk med små (~200 g) og store (~2–4 kg) fisk utsatt for varierende varighet og temperatur på ferskvannsbad, ulike termiske eksponeringstemperaturer, eller en sekvensiell behandling med bad etterfulgt av termisk eksponering. Fokus var i stor grad på fysiologisk respons, atferdsmessig respons, ekstern velferdstilstand, og for kombinasjonsbehandlingene, effektivitet i å fjerne hann- og hunnlus. En pilotstudie på salinitet bekreftet at regnbueørret tåler brakkvann opptil ~31 ppt uten negative effekter, men fullt sjøvann (34–35 ppt) hemmet vekst og økte risikoen for sår. Forsøk med ferskvannsbad viste at ørret tolererte eksponeringer opptil 4 timer med begrensede velferdskonsekvenser, men lengre varighet førte til vedvarende osmoregulatoriske forstyrrelser. Eksperimenter med termisk eksponering viste tydelige atferdsmessige stressresponser og stort innslag av likevektstap, særlig hos stor ørret hvor redusert relativ hjertestørrelse sannsynligvis begrenset toleransen. Flere varianter av kombinasjonsbehandling med ferskvannsbad etterfulgt av termisk eksponering ble testet, og viste at den kombinerte effekten forbedret avluseffektiviteten sammenlignet med hver behandlingstype isolert. Høyeste avlusningseffekt var ~84 % i eksperimentelle forsøk (ferskvann+32°C), med beskjedne og reversible fysiologiske effekter. Totalt sett viser funnene at regnbueørret krever tilpassede velferdsterskler som skiller seg fra atlantisk laks. Korte ferskvannsbad (2 timer) før varmeeksponering hadde samme avluseffekt som lengre bad (4 timer), men representerer en lavere velferdsbelastning. Kombinasjonsbehandlinger synes mer effektive enn enkeltbehandlinger, men krever nøye kontroll av varighet, temperatur og håndtering. Disse resultatene gir et grunnlag for eventuell evidensbasert godkjenning og optimalisering av avlusingsprotokoller for regnbueørret, og bidrar til både mer effektiv luskontroll og forbedret fiskevelferd i kommersiell akvakultur.
Effect of thermal and freshwater treatments on rainbow trout welfare, physiology, and infestation levels.
— Final report for AvlusØrret (FHF project 901869)
Report series:
Rapport fra havforskningen 2025-76
ISSN: 1893-4536
Published: 25.11.2025
Project No.: 14930-06
On request by: FHF
Reference: 901869
Research group(s):
Dyrevelferd
Subject:
Fiskevelferd,
Lakselus
Program:
Fremtidens havbruk
Research group leader(s):
Lars Helge Stien (Dyrevelferd)
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Robin Ørnsrud
Norsk sammendrag
Summary
The project aimed to investigate welfare impacts of single and combined delousing treatments for rainbow trout, and their efficacy at removing salmon lice. This included freshwater baths, thermal exposures, and their sequential application in rainbow trout (Oncorhynchus mykiss) to address critical knowledge gaps compared to Atlantic salmon. A comprehensive set of experiments were conducted to determine the species-specific response of trout to the treatments, including experiments on small (~200 g) and large (~2-4 kg) trout exposed to varying durations and temperatures of freshwater baths, different thermal exposure temperatures, or a sequential treatment of bath followed by thermal exposure. Focus was largely on physiological response, behavioural response, external welfare status, and for the combination treatments, delousing efficacy on male and female lice. A pilot salinity study confirmed that rainbow trout tolerate brackish water up to ~31 ppt without adverse effects, but full-strength seawater (34–35 ppt) impaired growth and increased risk of lesions. Freshwater bath trials showed that trout tolerated exposures up to 4 h with limited welfare consequences, but longer durations caused persistent osmoregulatory disturbances. Thermal exposure experiments revealed clear behavioural stress responses with frequent loss of equilibrium, particularly in large trout where reduced relative heart size likely constrained tolerance. A number of iterations of freshwater bath followed by thermal exposure were tested, which showed that the combined effect of them improved delousing efficacy compared to single methods. Maximum lice detachment reached ~84% in experimental trials (freshwater+32°C), with modest and reversible physiological effects. Overall, the findings demonstrate that rainbow trout require tailored welfare thresholds distinct from Atlantic salmon. Short (2 h) freshwater baths before thermal exposure had similar delousing effect as longer bath (4 h) but represent a lower welfare load. Combination treatments seem more effective than single treatments but require careful control of duration, temperature, and handling. These results provide a foundation for a possible evidence-based approval and optimisation of delousing protocols for rainbow trout, supporting both a more effective lice management and improved fish welfare in commercial aquaculture.
1 - Introduction
The aim of this project was to provide essential documentation of the welfare and delousing effect of freshwater and thermal treatments, and the combination of them, in rainbow trout (Oncorhynchus mykiss). At the beginning of the project, combination methods were not in widespread use for rainbow trout and the governing authority (Mattilsynet) was moving away from allowing welfare documentation for salmon to be transferred to trout. This project aimed to pave the way for new control strategies to be approved for trout that were not currently commercially available, optimised to their species-specific physiology, behaviour, and infection dynamics with salmon lice (Lepeophtheirus salmonis).
Considering the high production capacity of rainbow trout in Norway, very little fundamental knowledge has been published on the response of trout to freshwater bath or thermal exposure. In order to gain approval for sustainable use of these methods in the field, and to optimise delousing strategies to guarantee positive fish welfare status and delousing efficacy, we need to understand how they respond to iterations of the treatments, and how these responses change with other factors such as ambient temperature or body size. Although this project aimed to provide documentation so that farmers can accurately deploy sequential delousing methods (i.e. one method immediately followed by another), the results will contribute to fill the knowledge gap on trout physiological responses to treatment and provide interspecies comparisons between trout and salmon.
1.1 - Delousing methods and concerns when applying to rainbow trout
The use of these non-medicinal delousing treatments have been widespread in the salmon aquaculture industry (Overton et al. 2019). Recent experimental evidence indicates the effectiveness of freshwater bath, and freshwater bath followed by 34°C thermal exposure, on removing salmon lice (data from OptiDelouse FHF 901687; Bui et al. 2023), which supports the hypothesis of louse vulnerability to freshwater treatment (Guttu et al. 2024) and thermal shock (Nilsson et al. 2023). However their interaction with host species (Dalvin et al. 2020) may influence the louse’s tolerance to treatments. Other salmonid species have slightly different susceptibility to lice compared to salmon (Bui et al. 2018) and the innate differences in behaviour or morphology of rainbow trout could influence how they experience delousing efforts; for example, trout often exhibit stronger aversive behaviours to crowding and pumping, which may cause higher detachment rates of lice during these activities. On the other hand, the more viscous mucous layer observed in larger trout could provide a different protective environment for attached lice during exposure to treatments.
Rainbow trout farmers have based delousing strategies and operations on evidence of Atlantic salmon thresholds, as there is no data available for trout. However, as with Atlantic salmon, high mortality rates can occur after treatments with little understanding of the mechanisms causing poor tolerance to the procedure (Overton et al. 2019). The general experience is that mortality is typically higher in trout than salmon, especially in larger size classes (2-3 kg +) due to their heart health and sensitivity to stress. Expected responses to any stressors (such as exposure to freshwater bath or thermal exposure) are generally drawn from knowledge from Atlantic salmon, however trout are less likely to cope in the same way (e.g., Fevolden et al. 1993). For example, rainbow trout are often observed to have more pronounced rates of panic behaviour with handling operations, which would likely be exacerbated with longer treatment times. Further, risk of mortality is higher when trout have poor health status (e.g., wound prevalence, nefocalcinosis, ulcers, PRV-3, cardiac deformities, or having entered puberty), with observations of higher incidence of underlying or subclinical diseases following thermal treatments above 28°C. Acute mortality events have been reported in rainbow trout following freshwater treatment. A critical question for the industry is the tolerance limits of rainbow trout to freshwater exposure, and there is a glaring absence of data available on responses of trout to freshwater after seawater transfer.
1.2 - Verifying new delousing strategies for rainbow trout
The use of more than one method when treating fish could be more effective at removing lice, reducing the potential for development of population-level treatment resistance in lice. In Atlantic salmon, delousing efficacy of a freshwater bath can be supplemented by a thermal treatment (34°C) applied directly after, without further adverse welfare effects (OptiDelouse FHF project 901687). This could be adapted for rainbow trout, and trout producers have some anecdotal evidence that indicate the conditions that could maximise efficacy while retaining positive welfare in their fish. For example, they have experience good delousing effect when the temperature difference (ΔT°; from ambient sea temperature to thermal bath) is between 22 and 24°C; however, the general impression is that this effect on lice varies with season/sea temperature and size of fish. For welfare reasons, applying lower thermal treatment temperatures is desirable however previous studies indicate that louse detachment rate declines with decreasing thermal shock and that absolute treatment temperature is more important for detachment than ΔT° (Nilsson et al. 2023).
A solution to reduce thermal temperature without decreasing ΔT° is by cooling the temperature of the freshwater bath, which has been observed to produce a better delousing effect of mobile stages when followed by a thermal treatment (ΔT° = 22) (unpublished data, Are Nylund). Positive behavioural changes are also observed with cold FW baths, with a somewhat sedative effect on the fish (pers. comm. Bjørn Roth, Nofima). Yet, this needs to be investigated experimentally as there is also evidence for poorer tolerance of cold SW in rainbow trout compared to Atlantic salmon, where rainbow trout were ten times more likely to develop distended, water-filled stomachs compared to salmon (Rørvik et al. 2000).
The summary of background knowledge here is limited, which reflects the literature available for rainbow trout. There is a need for fundamental data on the response of trout to freshwater, thermal exposure, and mechanical stress in order to optimise commercial delousing strategies, particularly with relation to heart health, behaviour, welfare status, and physiological response. Many of these factors will have interacting effects – for example, larger trout have relatively smaller hearts which may correlate to their susceptibility to stress, while those with poor heart condition might find it harder to cope with stressful procedures (e.g., Bui et al. 2022). Thus, the differences in behaviour and physiology of trout from salmon requires their own documentation to ensure best practice operations.
1.3 - Project objectives and outcomes
There is little baseline data available on rainbow trout that can be used to improve fish welfare and delousing efficacy (and thus, potential resistance development in lice populations) with use of freshwater baths or thermal treatments. There is a pressing need to improve current industry practices as heavy mortalities are occasionally experienced after delousing events, where the reason for the loss is not completely understood. As such, the trout farming companies are in dire need of welfare and efficacy documentation so that they can optimise single method treatments, and so that sequential methods can be approved by Mattilsynet for commercial use. The group of industry representatives has quoted that “although our experiences with freshwater in interaction with thermal/flushing are positive for trout, and in part the same as for salmon, methods are currently not accepted. In order to gain acceptance for the methodology in the field and optimise delousing in terms of fish welfare and effect against lice, we also need more fundamental knowledge for trout”. Further, the mapping of more fundamental physiological responses will improve fish welfare across a range of commercial applications, taking into account environmental and other health factors.
The project’s objective was largely to develop optimal welfare and delousing efficacy recommendations for using freshwater baths alone or used sequentially with thermal treatments on rainbow trout, through evidence-based physical thresholds.
The subobjectives included mapping physiological and behavioural responses of trout to freshwater baths and thermal exposure, determining how responses changed with temperature (both ambient temperature and ΔT°), and determine delousing efficacy of sequential methods. We aimed to investigate whether these factors affect trout differently according to their body size (i.e. using >2 kg fish), and looked to commercial datasets to find preliminary evidence of transferability of our results.
1.4 - Project organisation
The project team comprised of researchers from the Animal Welfare group (Jonatan Nilsson, Angelico Madaro, Samantha Bui, Lars Helge Stien and Lars Eirik Myklatun) and the Reproduction and Developmental Biology group (Thomas Fraser). A group of industry representatives supported the project, gave insights into current practices and anchored the objectives to meet the existing knowledge gaps. This working group were represented by Avkavet Gulen, Osland Havbruk, Firda Seafood Group, Hofseth Aqua, Blom Fiskoppdrett, Svanøy Havbruk, Nordfjord Laks, Tombre Fiskeanlegg, and others. This project was funded by the Norwegian Seafood Research Fund (FHF), with a reference group consisting of representatives from industry and FHF.
Synthesis among other projects: AvlusØrret has been run in parallel with the project Welfare Severity (NFR 326980), in which welfare effects of various delousing treatments on salmon and rainbow trout, especially thermal treatment, have been studied. Several project members have been active in both projects, facilitating knowledge transition between the projects. Our experience from previous projects on delousing treatment of salmon, including IMR internal project 14930-02; TermVel (FHF 901649) and Optidelouse (FHF 901687) has contributed much to the design of set ups and analysis in AvlusØrret.
2 - General methods
All controlled experiments in this project were done in indoor tanks at Matre Research station, although the trout were kept in larger outdoor tanks for a period before being transferred to indoor tanks. Except for the salinity tolerance pilot (Section 3) all individuals were acclimated to seawater before experimental start. The freshwater supply in Matre is buffered to ~1 ppt salinity with seawater, but is in the following referred to as freshwater.
When trout were transferred between tanks fine meshed dip nets (< 10 mm) were used to minimize injuries on the fish (Moltumyr et al. 2024).
Welfare scoring was done with relevant indicators from the Laksvel scoring system (Nilsson et al. 2022).
Plasma was analysed in most experiments. Blood was drawn from the caudal vein using 2 ml heparinized syringes fitted with 23-gauge needles. Plasma was immediately separated by centrifugation at 13,000 rpm and at 4°C for 3 minutes. The uppernatant plasma was then transferred to a new tube, immediately frozen on dry ice and stored at −80°C until biochemical analyses were performed.
Plasma osmolality was measured by freeze point determination using 20 µl plasma subsamples with a Fiske 210 Micro-Sample Osmometer (Advanced Instruments). Other plasma parameters, including pH, lactate, and glucose, were measured from 65 µl subsamples using an ABL90 FLEX blood gas analyzer (Radiometer). This instrument is originally intended for fresh humane blood, but previous tests on salmon done in Matre indicate that most parameters are measured with relatively high precision also on fish plasma. However, pH seems to be underestimated. Reliably measuring true pH levels are challenging in fish blood. We therefore present pH levels given by the blood gas analyzer, but levels should be seen as relative differences between group rather than as absolute levels.
All experiments in this report were conducted at a certified facility for animal experimentation (Matre Research Station, facility number 110). Ethical use of experimental animals was requested in the application "Essential knowledge to develop delousing methods for rainbow trout" submitted by Samantha Bui, and was approved by the Norwegian Food Safety Authority (application ID number 30336).
3 - Pilot study to identify salinity tolerance
3.1 - Experiment 1: Response to salinity
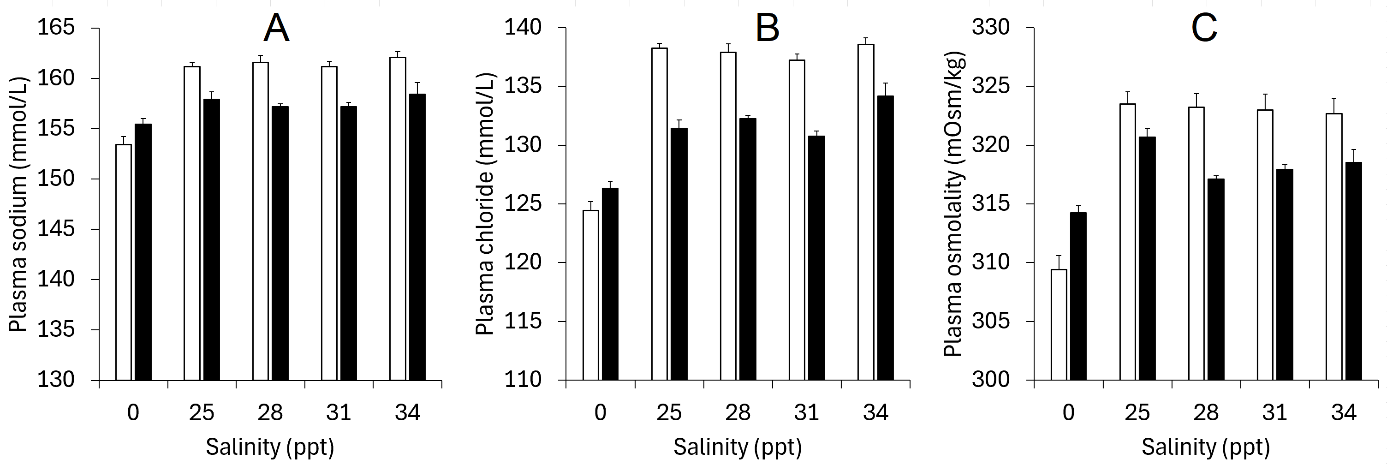
In preparation of initial trials in this project rainbow trout developed wounds and increased mortality after transfer to full-strength seawater (34-35 ppt). Seawater in combination with other stressors has in other studies (Fevolden et al. 2003) been shown to induce high mortality in rainbow trout of similar size as in our cases (~250 g). Since too low salinities would negatively affect salmon lice in later delousing efficacy trials, we needed to find a salinity window that could be tolerated by both rainbow trout and salmon lice. We therefore performed a pilot study where rainbow trout of 210 ± 28 g and 248 ± 12 mm (mean ± S.D) of the Aquagen strain were transferred from 2 m (4000 L) freshwater tanks to 1 m (400 L) tanks with either freshwater (control), 25, 28, 31 or 34 ppt salinity in quadruple groups of 24 individuals. Three fish/tank, i.e., 12 fish/salinity, were sampled for blood plasma, welfare scores, length and weight on days 1, 3, 7, 14 and 21. All fish in each tank were measured for weight and length on days 0 and 21. Only the 21 days specific growth rate (SGR) and plasma data from Day 1 and 21 are presented here.
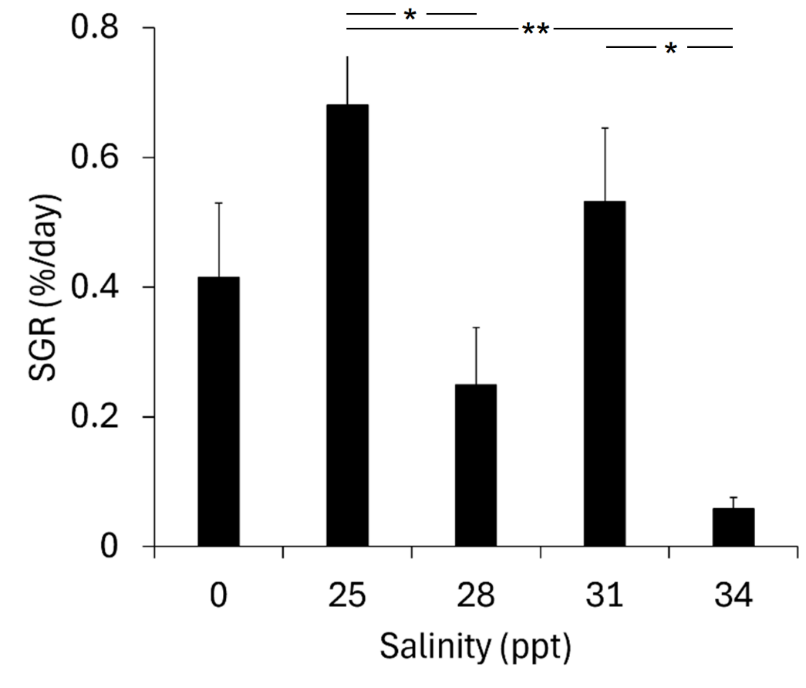
No mortalities that could be attributed to salinity occurred during the study (8 individuals in the freshwater control group died following an incident with gas hypersaturation in the freshwater supply). Although osmolality, Cl- or Na+ as expected was higher in saline water, these levels fell from day 1 to day 21, indicating that the trout were able to osmoregulate properly (Fig. 3.1), and no fish in any of the salinity groups had levels above normal ranges for salmonids (Noble et al. 2020). However, specific growth rate was considerably lower in the 34 ppt group, with almost no growth, than in the other groups, and significantly so for the 25 and 31 ppt groups (Fig. 3.2). Furthermore, 3 out of a total of 5 fish that developed wounds (score 1-2) during the study were in the 34 ppt group (and 1 in each of the 25 and 28 ppt groups).
Based on these observations we decided to use 30 ppt as standard salinity for seawater in the remaining trials in the project, as salinity at this level did not show any negative effects in our salinity trial and is sufficiently high for lice infection, as found in various earlier studies.
4 - Welfare effects of freshwater bath
4.1 - Experiment 2: Freshwater bath of small rainbow trout
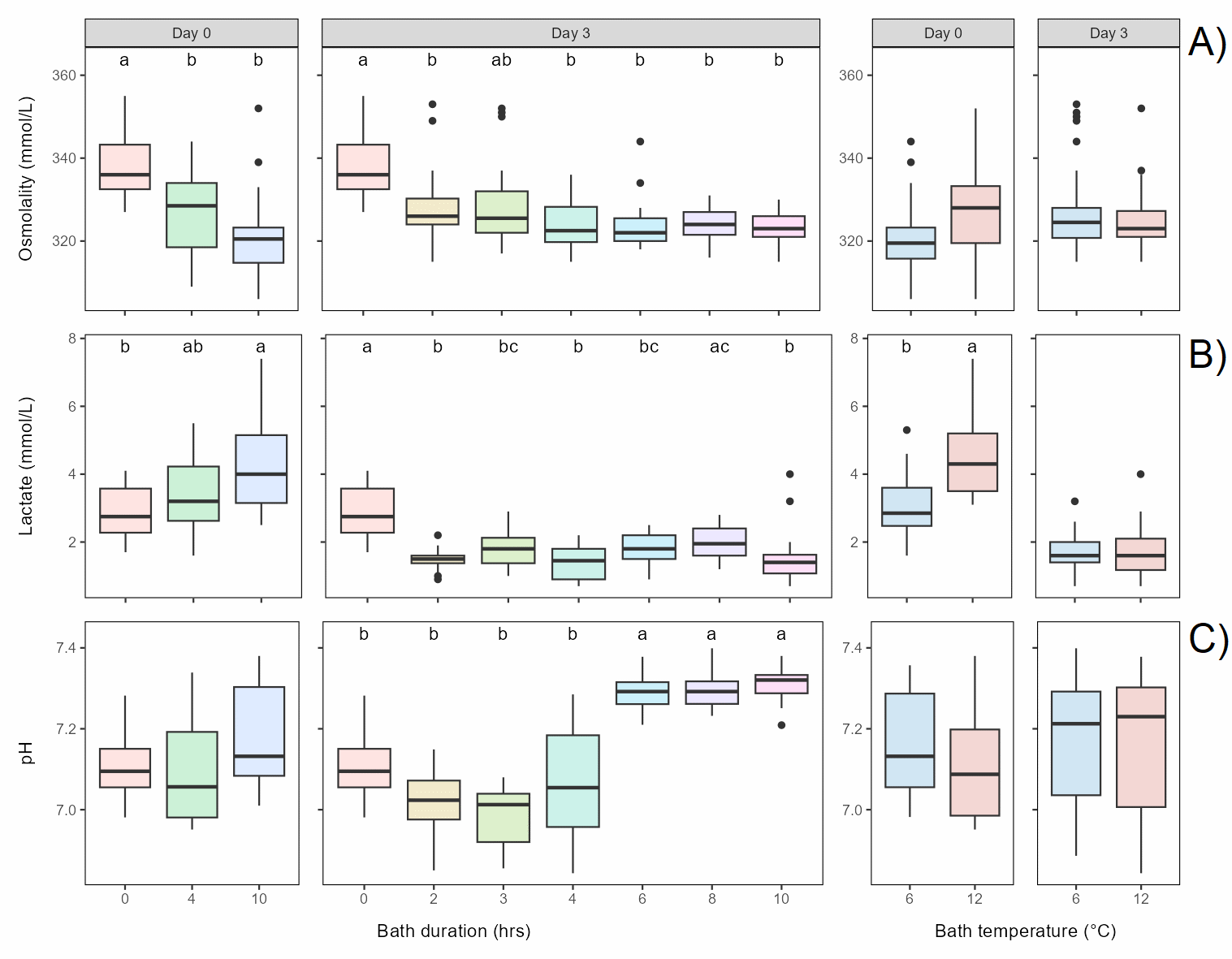
Rainbow trout of the Aquagen strain (287 ± 57 g, mean ± S.D) were acclimated to seawater (30 ppt) of 9°C for 4 weeks in 2 m tanks (4000 L). At start of the experiment, ten fish were netted directly from the seawater tank (baseline, time=0) and sampled for blood. Three hundred fish were transferred in groups of 30 fish/group to 3 m tanks (5600 L) filled with freshwater of either 6°C or 12°C and left there for 2, 3, 4, 6, 8 or 10 h. Twenty fish per group were thereafter transferred back to seawater in 1 m (400 L) tanks, with each freshwater bath duration × thermal temperature group in one tank, and left to recover for 3 days. For the 4 h and 10 h duration treatments 10 fish per group were also sampled immediately after the freshwater bath. On Day 3, the fish that had been returned to seawater were sampled for blood and welfare scores.
No fish in any group died during or in the 3 days following freshwater bath treatment.
Except for lactate on Day 0, no effect of temperature was found on any parameters measured, and data for the two temperature groups was therefore pooled for analysis of effects of bath duration.
4.1.1 - Plasma parameters
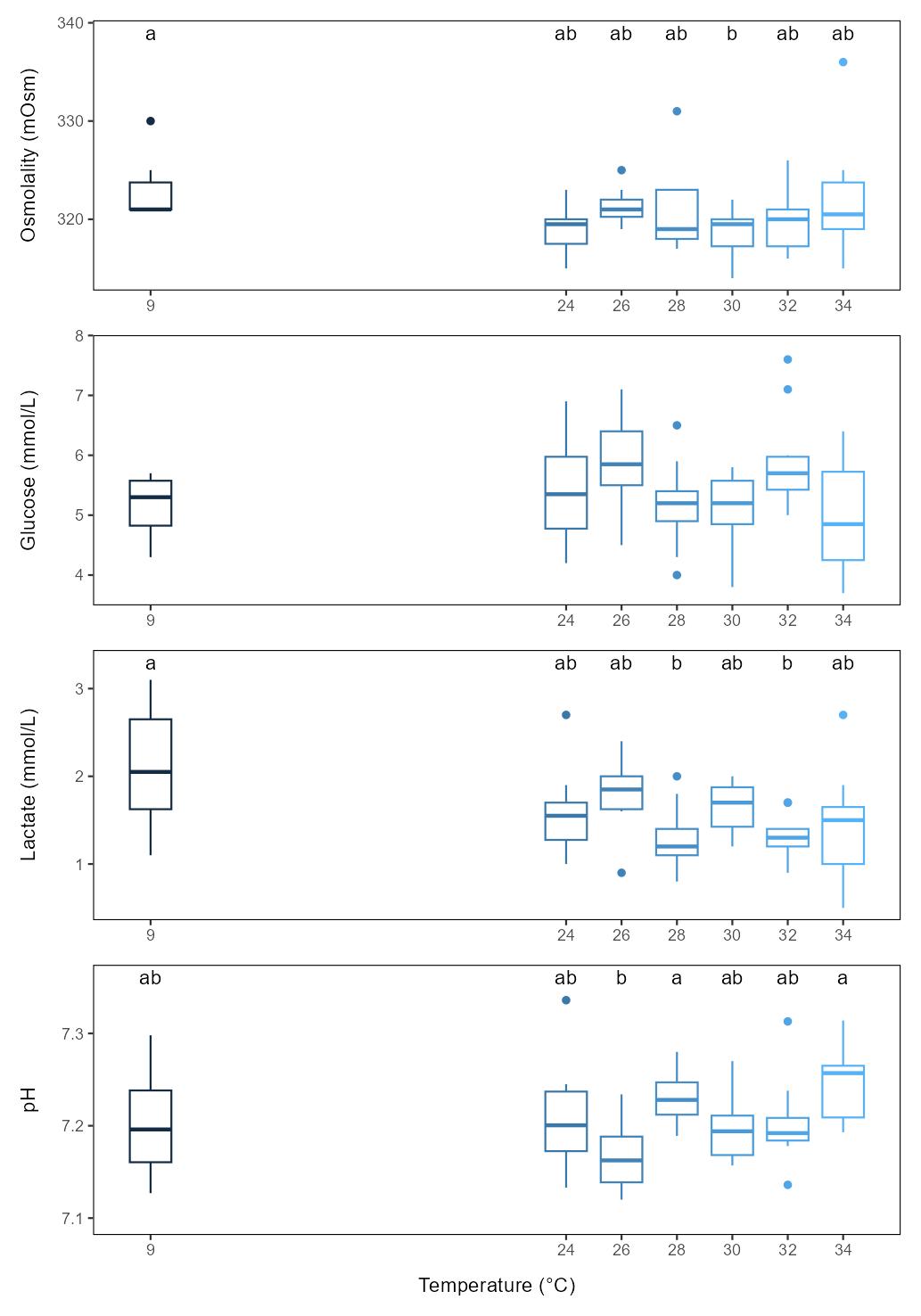
When sampled directly from freshwater, osmolality differed significantly from baseline levels (0 h) after 4 and 10 h freshwater bath, lactate only after 10 h, while pH did not differ from baseline (Figure 4.1).
When sampled after 3 days recovery in seawater, osmolality and lactate were still lower than baseline levels in most or all bath duration groups, while pH levels were similar to baseline in the 2-4 h bath duration groups but clearly increased from 6 h and beyond (Figure 4.1).
Physiological responses are thus not (only) acute but persist for days, and for pH the delayed effect was higher than the acute effect. Furthermore, baths with more than 4 h duration had higher effect than shorter baths both immediately after the freshwater bath and after 3 days, suggesting higher welfare impact of longer baths.
4.1.2 - Welfare scores
No differences in welfare score levels between the bath duration groups were found except for an unexplainable difference in scale loss between the 3 and 4 h bath duration groups, with less scale loss in the 3 h group. Percentage distributions of the scoring levels for the different welfare indicators are shown in Table 4.1.
| Bath duration | Scoring level | Scale loss | Skin haemorr. | Body wound | Snout damage | Eye opacity | Eye damage | Opercular damage | Gill damage | Fin damage |
| 2 h | 1 | 53 | 0 | 0 | 0 | 53 | 0 | 0 | 0 | 83 |
| 2 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 10 | |
| 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |
| 3 h | 1 | 10 | 0 | 0 | 0 | 35 | 0 | 3 | 0 | 85 |
| 2 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 13 | |
| 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |
| 4 h | 1 | 58 | 0 | 0 | 3 | 63 | 3 | 5 | 0 | 85 |
| 2 | 13 | 0 | 0 | 0 | 8 | 0 | 3 | 0 | 13 | |
| 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |
| 6 h | 1 | 28 | 0 | 0 | 0 | 60 | 0 | 0 | 0 | 83 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | |
| 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |
| 8 h | 1 | 43 | 0 | 0 | 0 | 50 | 0 | 3 | 0 | 90 |
| 2 | 8 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 8 | |
| 3 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | |
| 10 h | 1 | 15 | 0 | 0 | 0 | 65 | 0 | 0 | 0 | 80 |
| 2 | 8 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 20 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
4.2 - Experiment 3: Freshwater bath of large rainbow trout
Seawater acclimated rainbow trout of the Osland strain (2809 ± 586 g, mean ± S.D) were transferred from 5 m outdoor tanks to three 3 m tanks (5600 L) of 12°C 4 weeks before the start of the experiment. The day before freshwater bath the temperature was reduced to 9°C in order to have the same start temperature as in the experiment with small trout. At start of the experiment, 4 fish from each tank, in total 12 fish, were netted directly from the seawater tank (baseline, time=0), anaesthetised and sampled for blood. Thirty fish was thereafter transferred to each of three white transport tanks (1000 L) with freshwater of 6°C and kept there for a bath duration of 2, 4 or 8 h. Ten fish per bath duration group were anaesthetised and sampled for blood immediately after the freshwater bath. The remaining 20 fish per group were returned to seawater tanks and kept there to recover for 3 days. On Day 3 the water level in the tanks were lowered to allow netting of the fish, the fish were anaesthetised and sampled for blood and welfare scores.
Since the number of tanks was limited and no (or few) effects of bath temperature were found for small trout, only freshwater bath of 6°C was used in this experiment. The number of bath durations was also reduced.
No fish in any group died during or in the 3 days following freshwater bath treatment.
4.2.1 - Plasma parameters
When sampled directly after freshwater baths, osmolality was significantly lower than baseline levels (0 h) after 4 and 8 h freshwater bath while 2 h had no effect (Figure 4.2A). Glucose levels were higher than baseline in all bath groups with no difference between these groups (Figure 4.2B). Lactate levels in the 2 h group was higher than baseline and the 4 and 8 h groups Figure 4.2C), and this was reflected for the pH levels that was also only different in the 2 h group Figure 4.2D). We assume, however, that the elevated lactate levels and the linked reduction in pH in this group is a result of the relatively short duration between the stress of netting into the freshwater bath, which had still not recovered after 2 h, while it had so after 4 and 8 h.
When sampled after 3 days recovery in seawater, osmolality did not differ from baseline in any bath groups (Figure 4.2A). Glucose levels were slightly but significantly lower than baseline in all bath groups (Figure 4.2B), probably due to the 3 extra days with food omitted during recovery. Lactate levels were higher and pH lower than baseline in all bath groups (Figure 4.2C-D), but again this is probably an effect of recent muscular activity as the large tanks and fish required that water level was lowered and fish somewhat chased during netting prior to sampling.
4.2.2 - Welfare scores
Indicators skin haemorrhages, body wound, eye opacity, eye damage, opercular damage and gill damage, all fish were scored at level 0 (no deviation from normal). No differences in welfare score levels between the bath duration groups were found. For all bath groups combined all individuals were scored at level 1 or 2 for fin damage (22% and 78%, respectively) and scale loss (62% and 38%), and for snout damage at level 0, 1 or 2 (55%, 25% and 20%). No fish were scored at level 3 for any indicator. Percentage distributions of the scoring levels for the different welfare indicators per bath group are shown in Table 4.2.
| Bath duration | Scoring level | Scale loss | Skin haemorr. | Body wound | Snout damage | Eye opacity | Eye damage | Opercular damage | Gill damage | Fin damage |
| 2 h | 1 | 60 | 0 | 0 | 35 | 0 | 0 | 0 | 0 | 20 |
| 2 | 40 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 80 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 h | 1 | 65 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 20 |
| 2 | 35 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 80 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 h | 1 | 60 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 25 |
| 2 | 40 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 75 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
5 - Welfare effects of thermal exposure
5.1 - Experiment 4: Single thermal exposure of small rainbow trout
Seawater acclimated rainbow trout of the Aquagen strain (202 ± 33 g) were kept in groups of 20 individuals in 1 m (400 L) tanks containing sea water of 9°C. Two neighbour tanks of the same type were used as exposure tanks. These were filled with 20 cm seawater temperature of 9, 24, 26, 28, 30, 32 or 34°C, their lids lifted in front to allow smooth movement of fish in and out of the tank. A GoPro camera was attached to each of the tank lids to record the behaviour of the fish during exposure (Figure 5.1). Water was prepared by mixing seawater that had been heated to ~40°C in a transport tank with water of normal temperature (9°C) to the desired temperature.

Rainbow trout were netted one by one into the exposure tank (10 per tank per temperature) and left undisturbed for 30 s before they were transferred to tanks of 9°C again to recover for 3 days. In addition to recording of behaviour with the GoPro camera, we observed the fish “live” to see for loss of equilibrium (LOE) during exposure occurred in some individuals, as we have seen in previous studies with rainbow trout and which seems to be associated with heart arrythmia (Timmerhaus et al., unpublished data from Welfare Severity project NFR 326980). The intention of this was to keep LOE individuals in separate tanks during the recovery period following thermal exposure and compare heart size and shape in individuals that did and did not lose equilibrium. However, only one individual (at 26°C) lost equilibrium until the end of the 30 s exposure period, while a few other individuals seemed to be out of balance for some seconds but without complete LOE. All individuals of each exposure temperature were therefore kept in the same recovery tank, and hearts were not examined. Several (>10) fish in the 26-34°C groups were observed to lose equilibrium for approximately 10-20 s immediately after they were returned to 9°C, but since this was not expected no systematic recording of this was made and the frequency or duration of post treatment LOE could not be quantified.
The fish were sampled for plasma analysis and welfare scores 3 days after exposure.
Behaviour of the fish during thermal exposure was analysed from videos. Intensity of the behavioural response was scored on a scale from 0 to 3, where 0 represents almost no movements, 1 represents normal, calm behaviour (as expected when transferred to the same temperature), 2 represents a clearer response with higher swimming speed and many direction changes, often in combination with the head breaking the surface, while 3 represent a very strong behavioural response like rapid swimming, thrashing and collisions. Head shakes were also recorded. As reliable quantification of the number of head shake per fish was difficult, presence of head shakes was recorded as yes or no for the whole 30 s exposure period. Furthermore, the number of times the fish’s snout was breaking the surface was recorded.
No individuals at any temperature died during exposure or during the 3 days following exposure.
5.1.1 - Plasma parameters
When sampled 3 days after thermal exposure there was no clear dose-response effects of temperature on any plasma parameters, although there were some minor but significant differences between groups (Figure 5.2). For osmolality only the 30°C group differed from the control group, but not from the other temperature groups. Glucose levels did not differ between any groups. For lactate only the 28 and 32°C groups differed from the control group, but not from the other temperature groups. For pH no thermal group differed from the control, while the 28 and 34°C groups differed from the 26°C group.
5.1.2 - Welfare scores
For all indicators no or few individuals were scored at level 2-3 (Table 5.1). For some indicators a large proportion of the fish were scored at level 1 but with no clear relationship with temperature, suggesting that these deviations were present before treatment and/or inflicted by handling.
| Exposure temperature | Scoring level | Scale loss | Skin haemorr. | Body wound | Snout damage | Eye opacity | Eye damage | Opercular damage | Gill damage | Fin damage |
| 9°C | 1 | 100 | 0 | 0 | 0 | 80 | 0 | 10 | 90 | 50 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24°C | 1 | 100 | 0 | 0 | 0 | 80 | 0 | 5 | 65 | 40 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 26°C | 1 | 100 | 0 | 0 | 0 | 60 | 0 | 0 | 85 | 65 |
| 2 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 28°C | 1 | 100 | 0 | 0 | 0 | 74 | 0 | 11 | 100 | 53 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 30°C | 1 | 100 | 0 | 0 | 0 | 48 | 0 | 5 | 95 | 71 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | |
| 32°C | 1 | 100 | 0 | 0 | 0 | 55 | 0 | 5 | 90 | 65 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | |
| 34°C | 1 | 95 | 0 | 0 | 0 | 75 | 5 | 0 | 85 | 60 |
| 2 | 5 | 0 | 0 | 0 | 10 | 0 | 0 | 5 | 5 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
5.1.3 - Behaviour
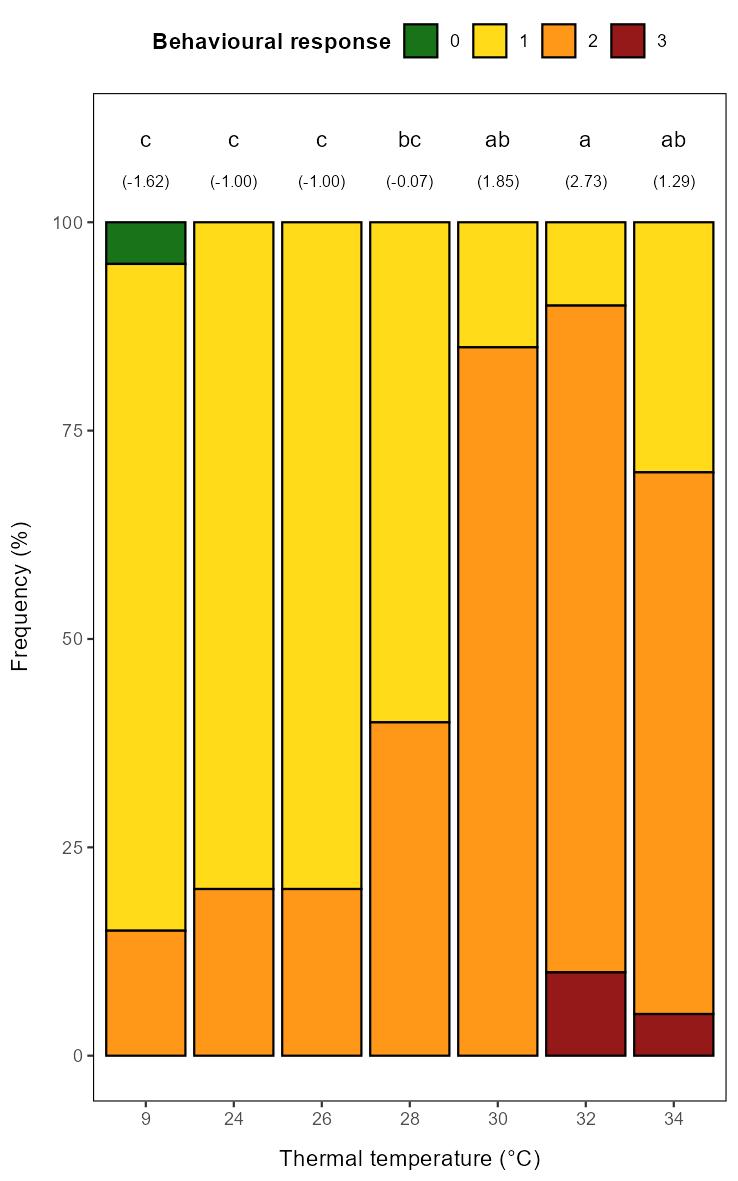
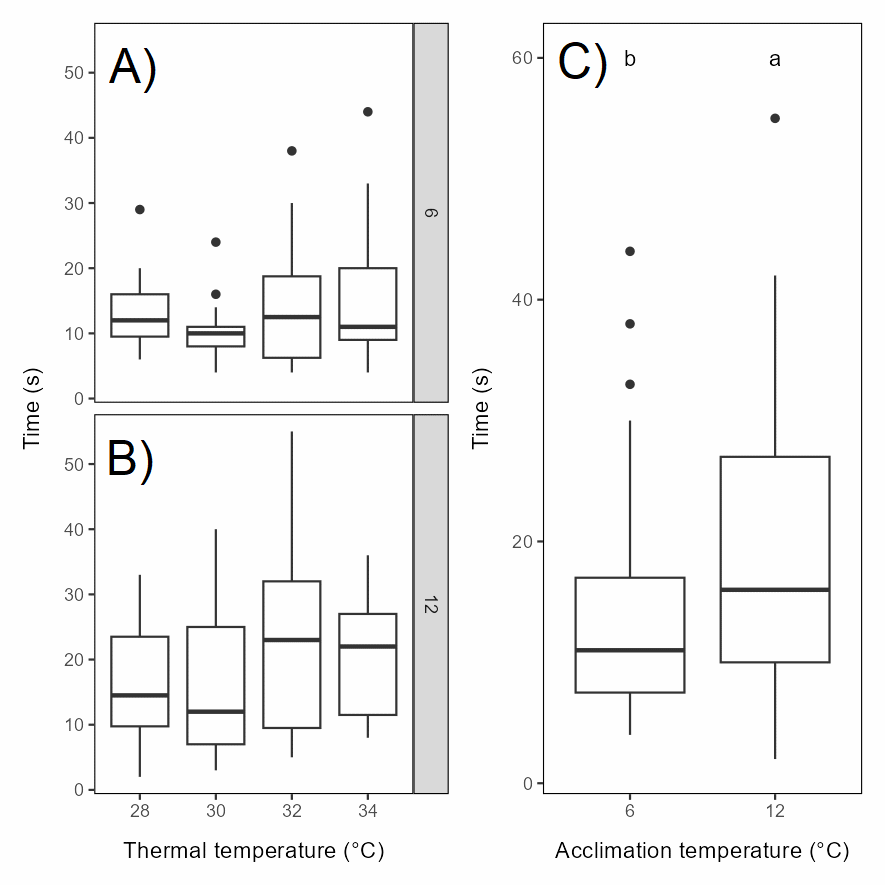
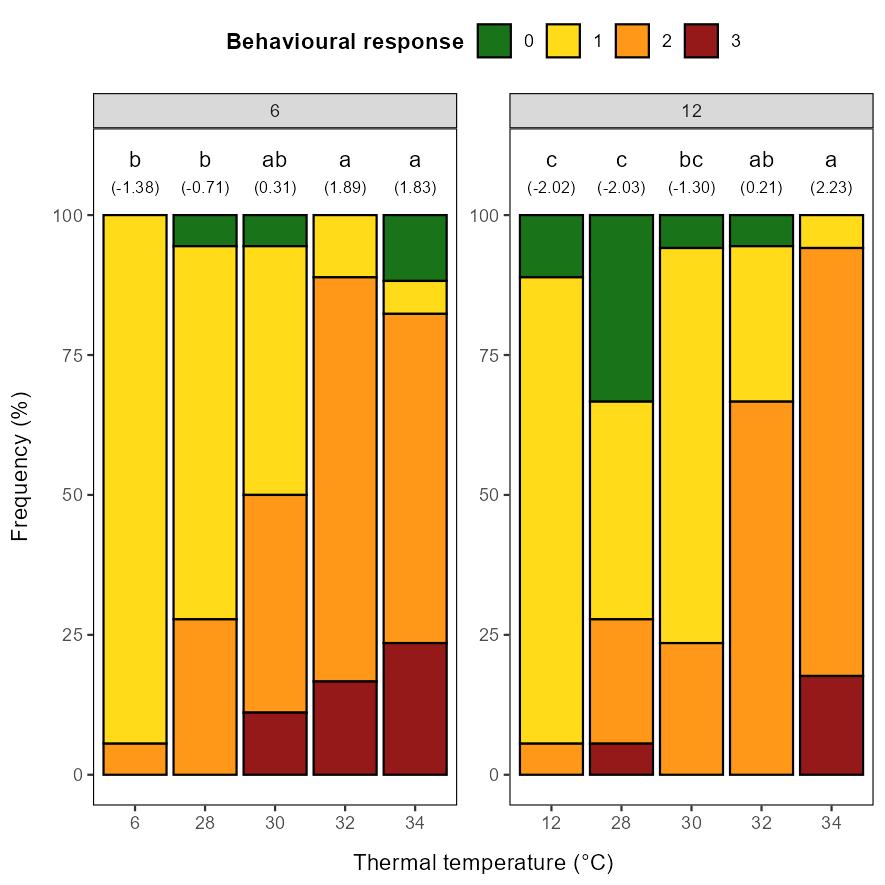
Our general impression of the behavioural response was that it was less strong than what we have previously observed in salmon at similar size at similar temperatures (Nilsson et al., 2019). Still, the intensity of the response (score 0-3) increased with temperature, with the distribution of scores significantly different in the 30-34°C groups than the control (9°C) and 24-26°C groups, with the 28°C group as an intermediate (Figure 5.3). The most intense response, score 3, was only observed in the 32-34°C groups.
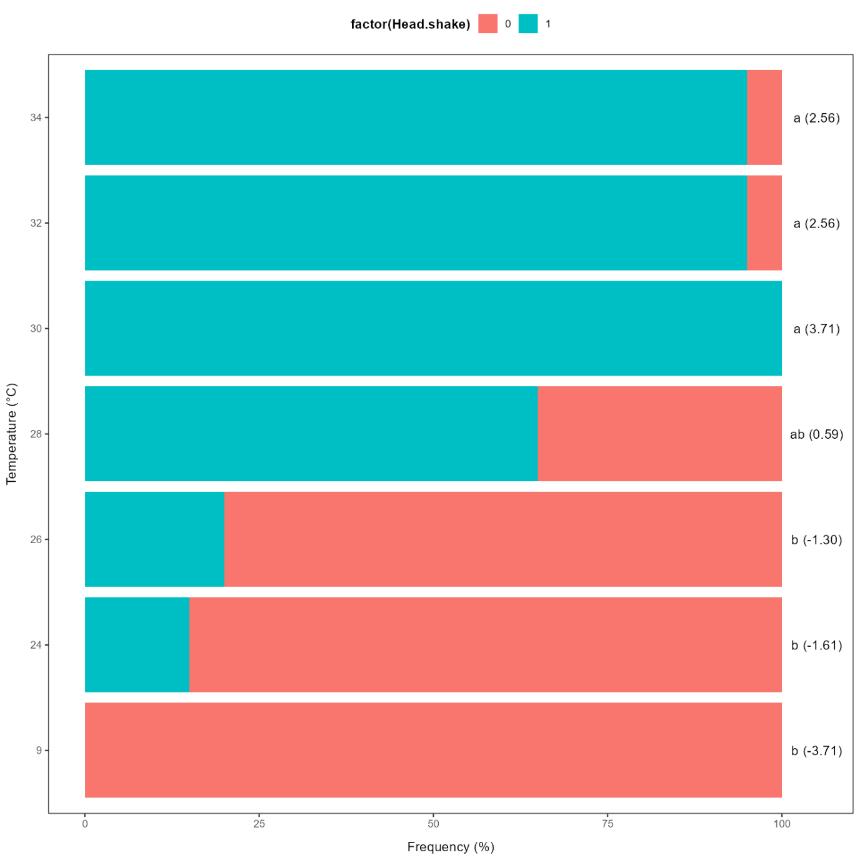
Head shakes were never observed in the 9°C control group and only for few fish in the 24-26°C groups, while the majority of the individuals in the 28-34°C groups performed head shakes (Figure 5.4). As for the behavioural response, the 9-26°C groups differed significantly from the 30-34°C groups, with the 28°C group as an intermediate.
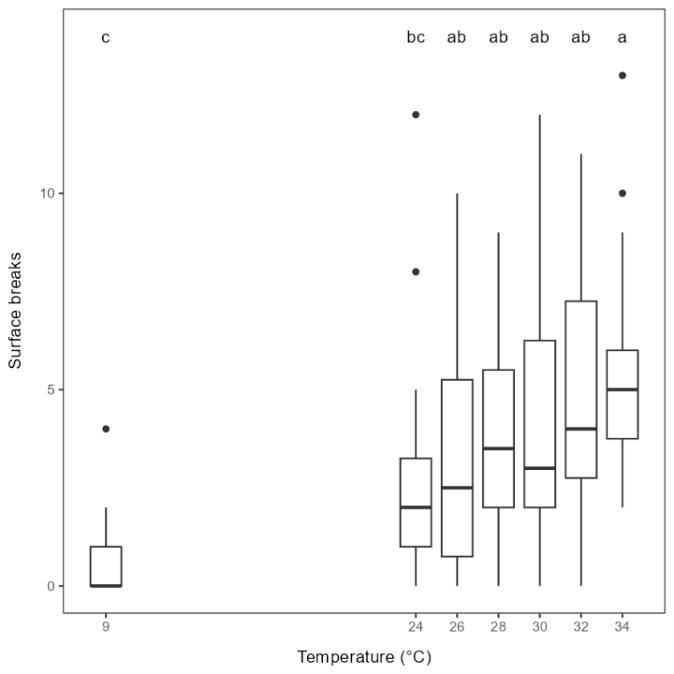
Rainbow trout exposed to warmer water appeared to swim closer to the surface than when exposed to lower temperatures. Swimming depth was not possible to quantify since the fish were only video recorded from above. However, the number of times that the snout was breaking the surface was higher at higher temperatures, and significantly higher than in the 9°C control group for all temperature from 26°C and above (Figure 5.5). This reflects the more near-surface swimming observed at higher temperatures. Surface breaks did not necessarily have to be associated with burst swimming or jumping, also relatively calm swimming resulted in more surface breaks at higher temperatures.
5.2 - Experiment 5: Single thermal exposure of large rainbow trout
Seawater acclimated rainbow trout of approximately 3 kg of the Osland strain, origin from the same original group as used for freshwater bath of large rainbow trout (Section 4.2), were used for this experiment, and kept in the same 3 m (5600 L) tanks. After the freshwater trial, 60 fish that had not been bathed in freshwater remained in each of three tanks, and the fish were acclimated to 6°C for 3 weeks before the thermal trial. Food was omitted from the day before the thermal exposure. Thermal (or control) exposure of 90 individuals, 30 from each stock tank, was done on 29 October 2024. The following day the water temperature in the stock tank was increased to 12°C, and the remaining fish were exposed in the same manner one week later (5 November). Thus, trout were exposed to the same thermal temperatures at different acclimation temperatures, i.e., different ΔT° for a given exposure temperature. The mean ± S.D weight and length of the trout was 2883 ± 600 g and 55 ± 4 cm for the 6°C acclimation group, and 2958 ± 527 g and 54 ± 4 cm for the 12°C acclimation group.
Due to the large size of the fish exposure with free swimming in open tanks, as done for small rainbow trout (Section 5.1) was not feasible as it would require large exposure tanks with little possibility to control exposure duration since the fish would be difficult to net out. The trout were therefore exposed in a smaller container.
Exposure procedure: The water level in the stock tank was lowered to ~45 cm depth and kept at that level with a flowthrough rate of 90L/min. Rainbow trout were netted out one at the time and transferred to a basket that was lowered into a transportation tank (60×60 cm) filled with water of the desired temperature, i.e., acclimation temperature as control (6 or 12°C), 28, 30, 32 or 34°C. After 30 s, the basket with the fish in was lifted over to a nearby transportation tank of the same type containing water of the acclimation temperature and transported to another 3 m tank, into which the basket was lifted and the fish released for 3 days recovery before final sampling (Figure 5.6). The basket and extra transportation tank were used to avoid unnecessary netting and air exposure that could have contributed to additional stress and injuries, especially since the fish were large and distances to recovery tanks relatively long (up to ~15 m). One recovery tank was used for each temperature group.
The fish were video recorded by a handheld GoPro camera from introduction to the thermal bath until it showed controlled swimming in the recovery tank, so that fish with loss of equilibrium (LOE) could also be analysed for individuals that were still out of equilibrium at the end of thermal exposure, or that lost equilibrium after return to acclimation temperature (as observed for small trout, Section 5.1). Two fish were subsequently exposed before the temperature was changed up or down 2°C or to the acclimation temperature. In total 18 individuals per acclimation group were exposed to each temperature.
No individuals at any temperature died during exposure or during the 3 days following exposure.
After 3 days of recovery the fish were sampled for size and welfare scores. The fish in the 12°C acclimation group was also sampled for heart size in order to evaluate the general heart health of the group, as LOE during exposure was frequently occurring in this experiment and LOE appears to be related to heart arrythmia (Timmerhaus et al., unpublished data). We could not correlate heart size with LOE since all fish per temperature group, both LOE and none-LOE fish, were kept in the same recovery tank, and since the individuals were not ID tagged, we could not separate LOE fish from none-LOE fish in the recovery tank.

5.2.1 - Welfare scores
For indicators skin haemorrhages, body wound, eye opacity, eye damage, opercular damage and gill damage, most fish were scored at level 0 (no deviation from normal), and only a few at level 1. For indicators scale loss, snout damage and fin damage, a larger proportion of the fish were scored at level 2, but with no clear relationship with temperature, suggesting that these deviations were present before treatment and/or inflicted by handling. Of all indicators combined, only two individuals were scored at level 3, one for opercular damage and one for snout damage. Percentage distributions of the scoring levels for the different welfare indicators per bath group are shown in Table 5.2.
| Exposure temperature | Scoring level | Scale loss | Skin haemorr. | Body wound | Snout damage | Eye opacity | Eye damage | Opercular damage | Gill damage | Fin damage | |
| 6°C acclimation temperature | 6°C | 1 | 83 | 0 | 0 | 61 | 6 | 0 | 0 | 6 | 50 |
| 2 | 17 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 50 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 28°C | 1 | 72 | 0 | 6 | 61 | 6 | 0 | 0 | 11 | 72 | |
| 2 | 28 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 28 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 30°C | 1 | 94 | 6 | 0 | 39 | 6 | 0 | 6 | 6 | 56 | |
| 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 32°C | 1 | 61 | 0 | 6 | 39 | 0 | 0 | 11 | 11 | 39 | |
| 2 | 39 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 61 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 34°C | 1 | 53 | 0 | 0 | 24 | 12 | 0 | 0 | 29 | 6 | |
| 2 | 47 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 94 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | ||
| 12°C acclimation temperature | 12°C | 1 | 100 | 6 | 6 | 50 | 11 | 0 | 0 | 0 | 89 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 28°C | 1 | 61 | 0 | 6 | 56 | 44 | 0 | 0 | 6 | 78 | |
| 2 | 33 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 22 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 30°C | 1 | 89 | 6 | 0 | 44 | 17 | 11 | 0 | 0 | 67 | |
| 2 | 6 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 28 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 32°C | 1 | 78 | 11 | 11 | 44 | 22 | 0 | 6 | 11 | 78 | |
| 2 | 17 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 17 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 34°C | 1 | 78 | 11 | 22 | 61 | 22 | 11 | 0 | 6 | 61 | |
| 2 | 17 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 39 | ||
| 3 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
5.2.2 - Behaviour
Videos were analysed for time to LOE, duration of LOE, i.e., from when LOE occurred to the fish started to swim again, and behaviour score (0-3, as described in Section 5.1). For LOE fish the behaviour score was based on the response before the individual lost equilibrium and could therefore be given a high score even if it was in motionless LOE during most of the exposure duration. As the large trout were exposed in a small container/basket with restricted possibilities for free swimming, analysis of detailed behaviours like head shakes and surface breaks was not possible.
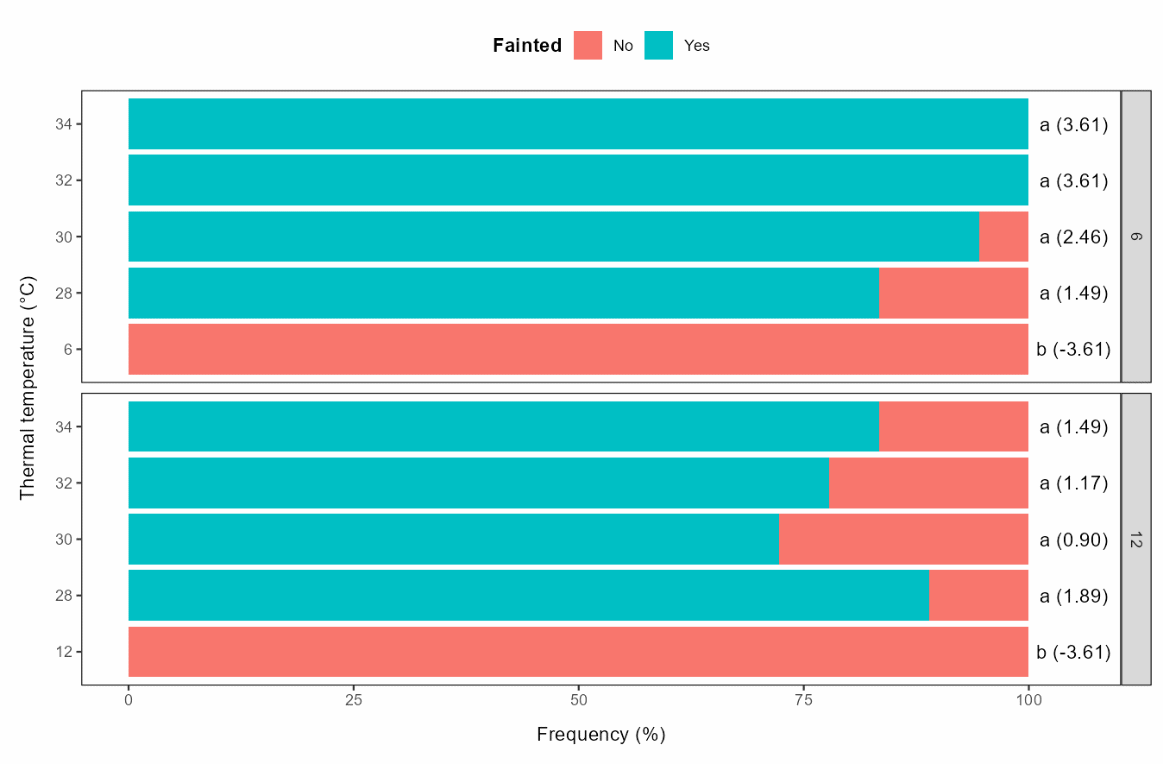
Except for the control temperatures, most individuals lost equilibrium, either during (66 and 48 for the 6 and 12°C acclimation groups, respectively) or after (2 and 11) exposure. There were no significant differences in the proportion of fish that lost equilibrium between exposure temperature groups, except for the control groups (Figure 5.7).
For the individuals that lost equilibrium, the time before LOE occurred after introduction to the thermal bath did not differ between exposure temperatures within the acclimation groups, while LOE occurred somewhat earlier for rainbow trout acclimated to 6°C (Figure 5.8).
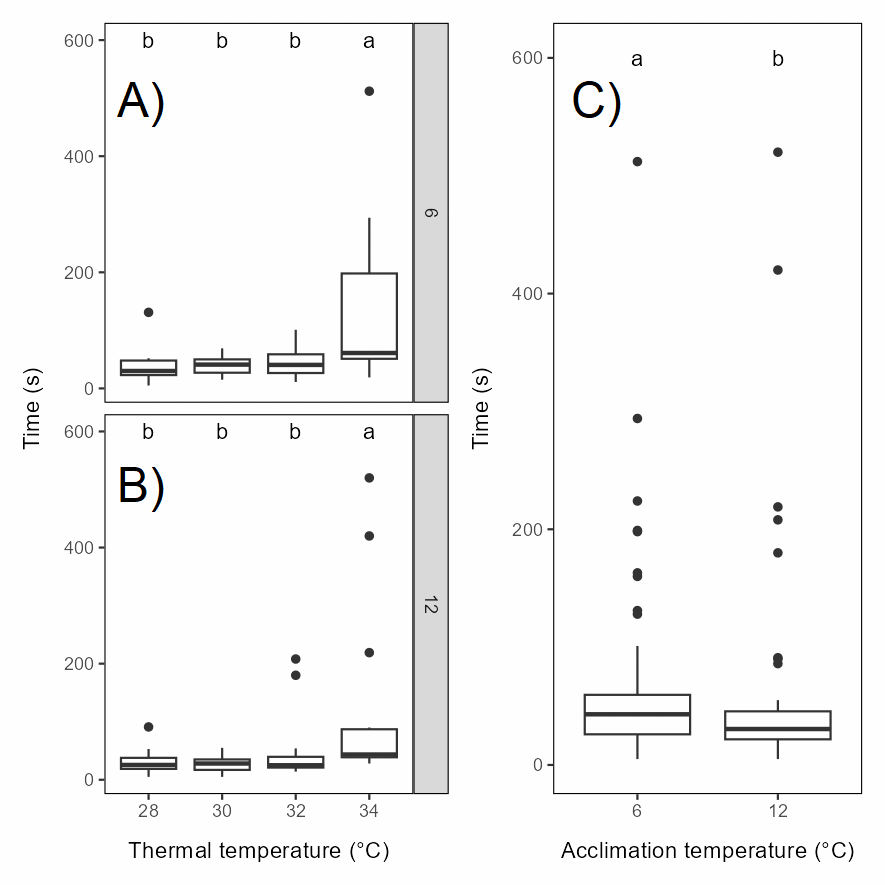
The duration of the LOE period did not differ between the 28, 30 and 32°C groups, while it was significantly longer in the 34°C group in both acclimation groups, with some individuals being out of equilibrium for more than 8 minutes after they were removed from the thermal bath. The LOE period was generally somewhat longer for rainbow trout acclimated to 6°C (Figure 5.9).
The behaviour score of rainbow trout generally increased with exposure temperature, with the score distribution significantly different in the 32 and 34°C groups than in the control and 28°C groups, with 30°C groups as an intermediate, for both acclimation temperatures (Figure 5.10). Still, some individuals were scored at level 3, i.e. a very strong response, also in the 28 and 30°C groups.
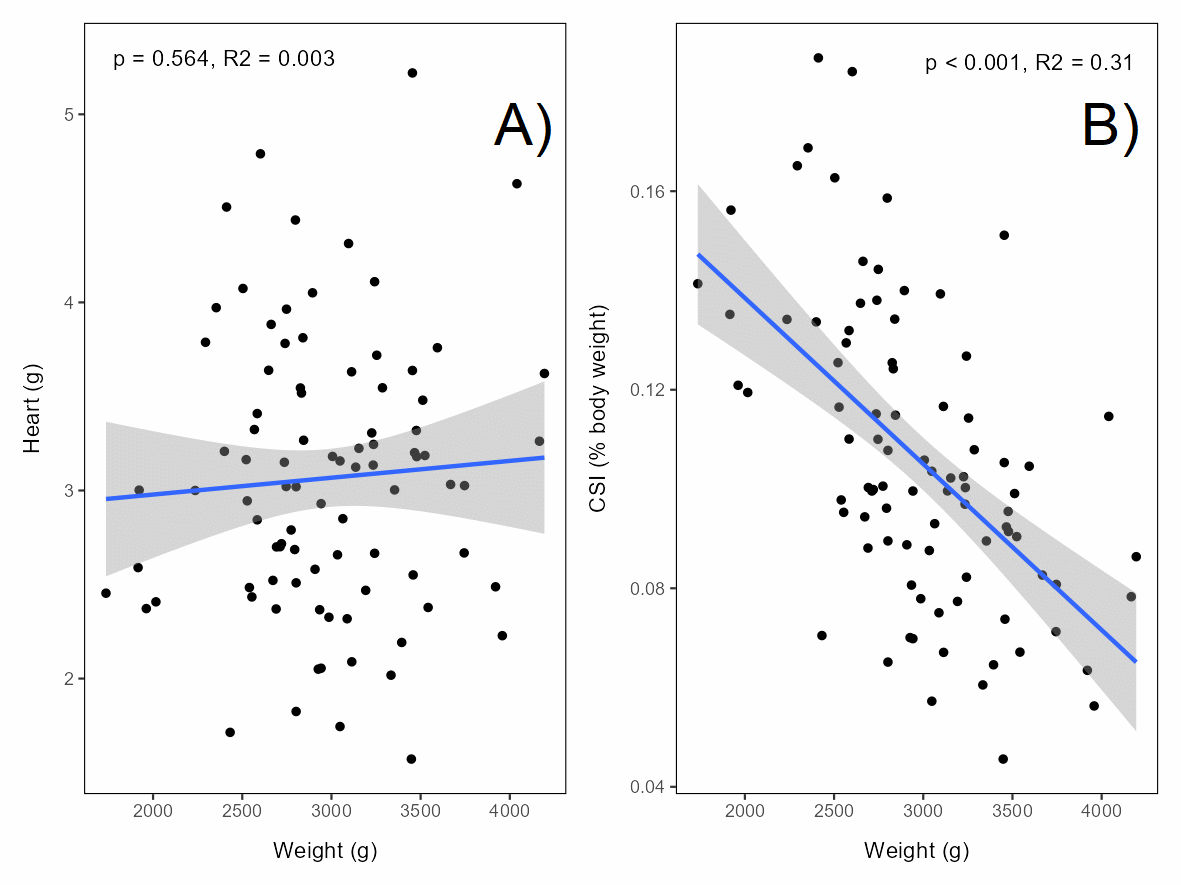
The absolute weight of the hearts (sampled from all individuals in the 12°C acclimation group) was not correlated with the weight of the fish (Figure 5.11A), and accordingly the relative heart weight (cardiosomatic index, CSI) declined strongly with fish weight, with relative heart weight of the largest individuals being ~half of that of the smallest individuals (Figure 5.11B).
6 - Delousing efficacy and welfare effects of combination treatments – Freshwater bath followed by thermal exposure
6.1 - Experiment 6: Methods
Rainbow trout of the Aquagen strain (1947 ± 330 g, mean ± S.D.) were held in groups of 10-11 fish in twenty-seven 1 m (400 L) tanks, in total 290 individuals. The tanks contained seawater (30 ppt) at 10°C.
Adult female lice and their eggstrings were collected from sites around Sognefjorden, and incubated at 10°C (following Hamre et al. 2009). Around two days after the larvae had moulted to copepodids, they were enumerated and divided to provide an infection pressure of 30 copepodids per fish, among the 27 tanks. To infect fish, water level and flow were reduced, and copepodids added. Oxygen conditions were monitored to ensure it remained >80%, and fish were occasionally stimulated to create movement. After 30 minutes, water flow was restored to the previous level. Infections occurred 54-58 days prior to delousing, whereby all lice were adults at the time of treatment.
Combinations of bath treatments and thermal treatments were manipulated to find how bath salinity, bath temperature, bath duration and thermal temperature affect the detachment effect on adult salmon lice. Since the number of possible combinations is almost infinite, we decided to use an “industry standard” that represent a realistic combination used at farms and manipulate one (sometimes more) variable at the time from this to see if the detachment and/or welfare effect change. Based on discussions with the project’s industry partners and reference group, it was decided that a 6°C freshwater bath of 4 h duration followed by a 30 s thermal exposure to 30°C would be a realistic “industry standard”. The manipulations of combinations resulted in 9 different treatment groups, described in Table 6.1. Five groups (in triplicates) were treated 2 December 2024 and the remaining four groups the following week, 9 December 2024.
All fish in each tank were netted over to a transport tank and transferred to one similar to their original tank but filled with bath water, i.e., freshwater or seawater of 6°C or 10°C, and kept there for 0 h (procedure controls that were transferred back to the original tank after a few minutes), 2 h or 4 h (Table 6.1). The fish were thereafter netted out for individual exposure to thermal treatment in a transport tank. In the first round of exposure on 2 December it was not possible to reliably collect lice that detached in the bath tank since lice could be evacuated through the drain. The following week the drains were sealed and the number of lice left in the tank after the fish had been removed from the bath was counted. We assume that the proportion of lice that detached in the freshwater and seawater baths was approximately the same also in the first week.
The thermal treatment was done by transferring fish one by one directly from the bath tank to a basket lowered in a transport tank containing water of 10°C, 28°C, 30°C or 32°C. Higher exposure temperatures was not used in this experiments due to the long loss of equilibrium (LOE) durations observed at 34°C in WP1 (Section 5.2). A fine mesh lining inside the basket captured lice that detach during thermal treatment (Figure 6.1). After 30 s exposure the fish was taken up and anesthetized and number of male and female lice on the fish and in the basket was counted.
The number of lice on each fish before thermal treatment is the sum of lice on the fish and in the basket after treatment, and lice detachment calculated as 100×(detached)/(before treatment). Detached lice could only be linked to individual fish in the thermal treatments where fish were treated individually. Therefore, in the statistical tests delousing effects of the combination treatments was assessed as the effect of the thermal treatment rather than of the entire combination treatment (i.e., bath, handling and thermal). Estimated additional loss (for FW_6°C_4 h and SW_10°C_0 h) during bath was however also assessed and described below. The fish were therafter returned to their original tank and left to recover for 3 days before they were anesthetized and sampled for blood, welfare scores and again counted for lice to see for delayed detachment of lice, i.e. during the 3 days in seawater. Since the fish were not ID tagged, delayed lice loss could not be assessed on individual level but at treatment group level. Estimation of overall detachment, i.e. detachment during bath + detachment during thermal treatment + delayed detachment, was done at treatment group level.
|
Bath salinity |
Bath temperature (°C) |
Bath duration (h) |
Thermal temperature (°C) |
Treatment description |
|
FW |
6 |
4 |
30 |
"Industry standard" |
|
FW |
6 |
2 |
30 |
Reduced bath duration |
|
SW |
6 |
4 |
30 |
Control bath salinity |
|
FW |
10 |
4 |
30 |
Control bath temperature |
|
FW |
6 |
4 |
10 |
Freshwater single |
|
SW |
10 |
0 |
30 |
Thermal single |
|
FW |
6 |
4 |
28 |
Reduced thermal temperature |
|
FW |
6 |
4 |
32 |
Increased thermal temp |
|
SW |
10 |
0 |
10 |
Full procedural control |

6.2 - Experiment 6: Welfare effects
6.2.1 - Plasma parameter levels after 3 days of recovery
Rainbow trout were sampled for plasma after 3 days of recovery in seawater following the treatment.
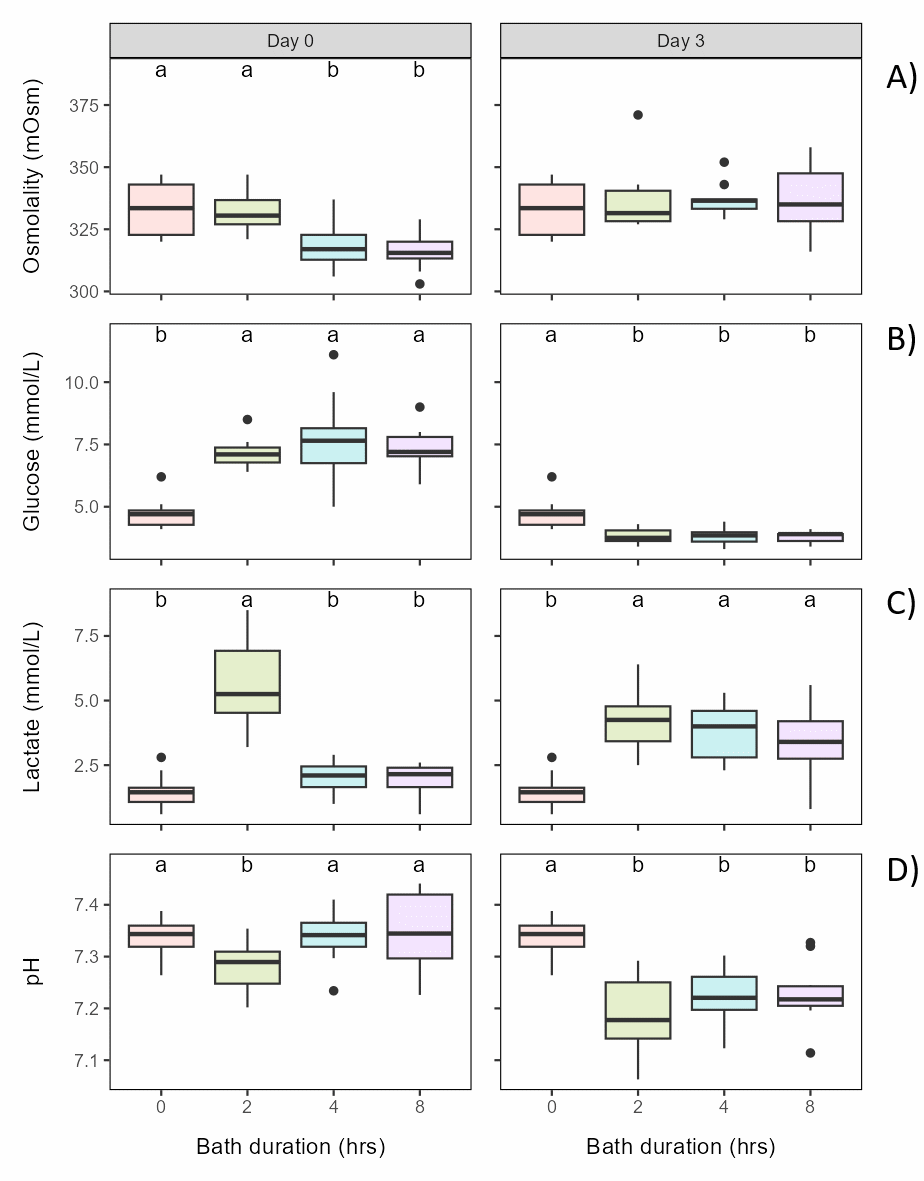
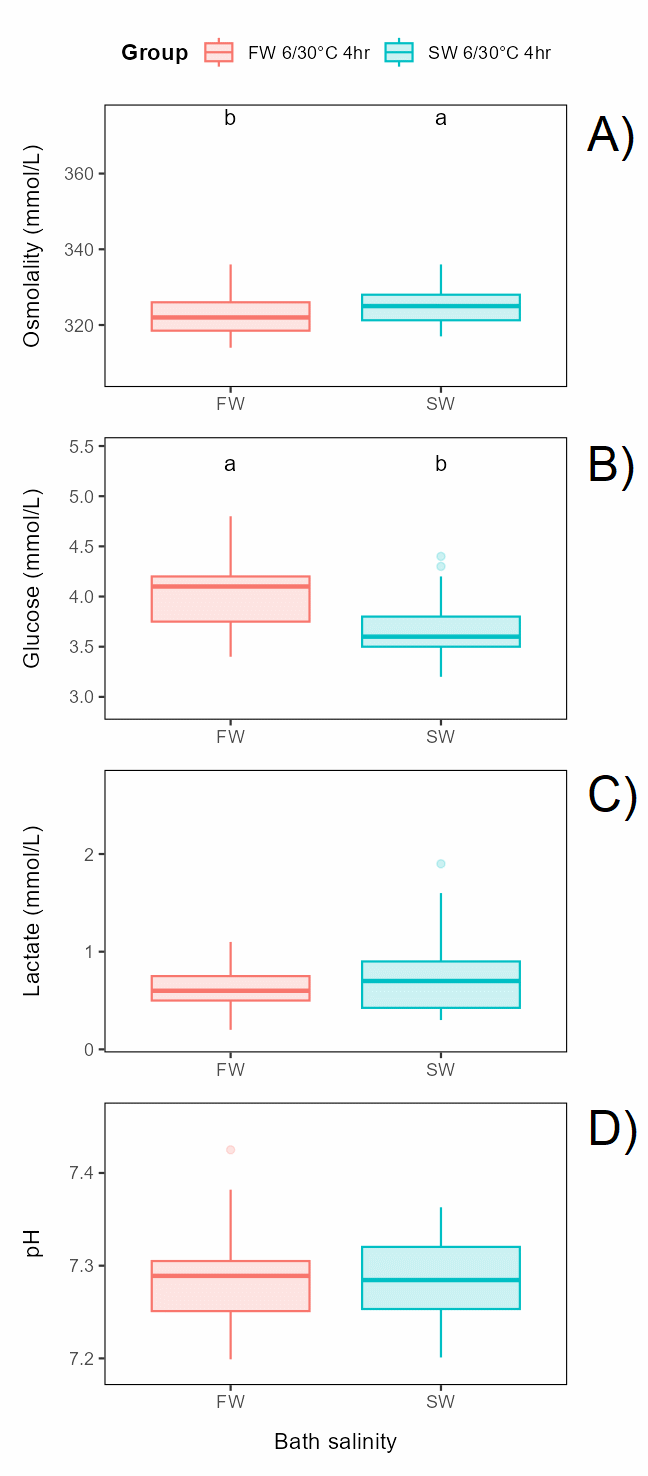
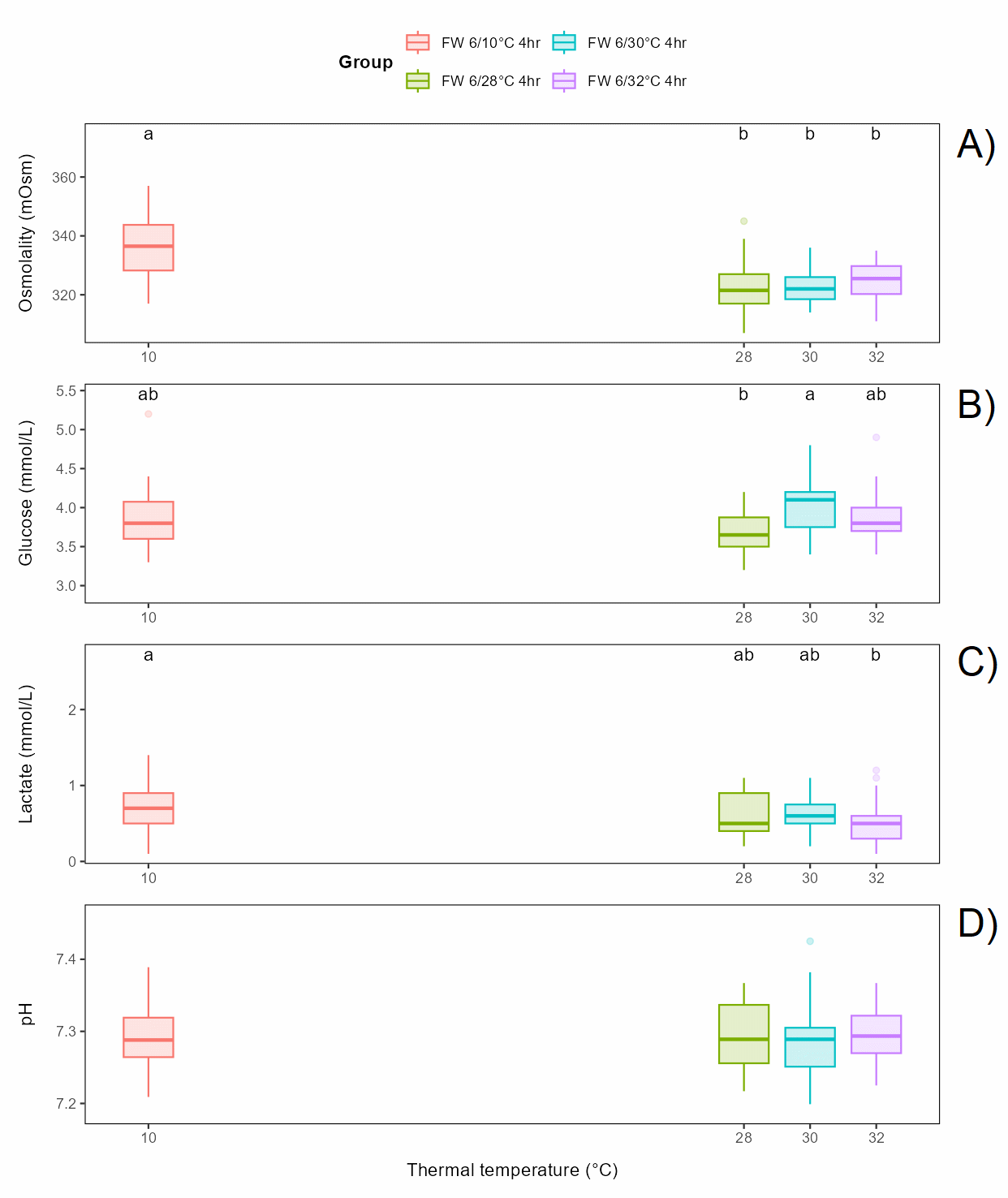
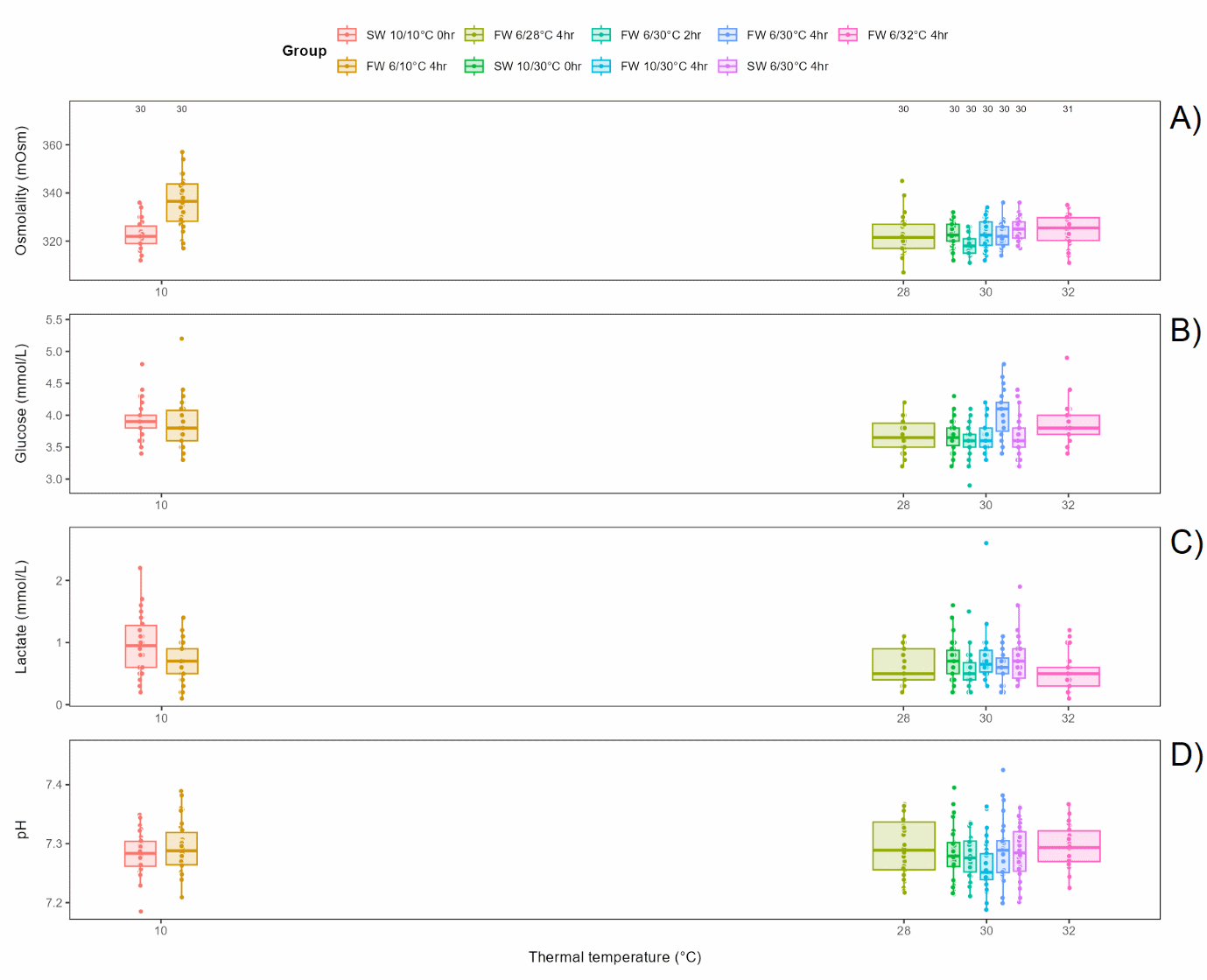
Bath salinity (fresh or seawater of 6°C for 4 h) before exposure to 30°C for 30 s had a small but significant effect on osmolality and glucose, but not on lactate or pH (Figure 6.2).
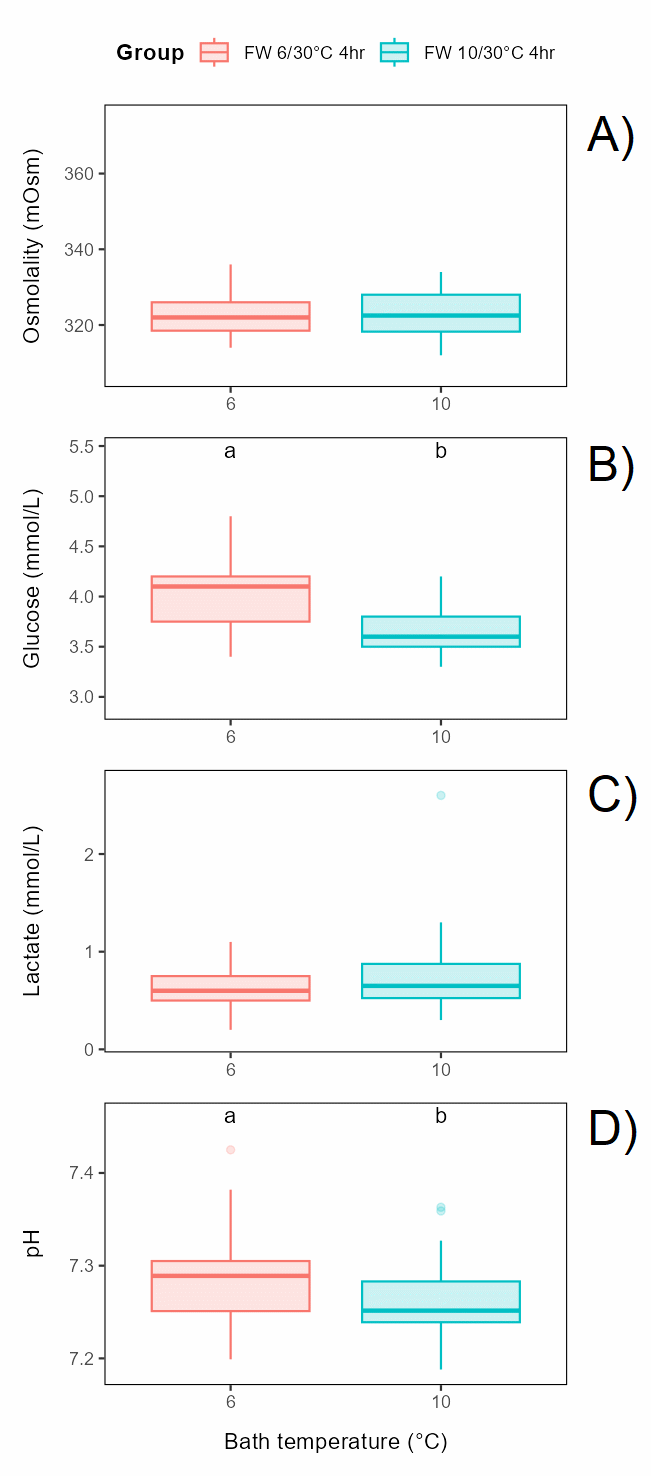
Bath temperature (freshwater of 6 or 10°C for 4 h) before exposure to 30°C for 30 s did not affect osmolality or lactate, while glucose and pH had slightly but significantly lower levels in the 10°C group effect on (Figure 6.3).
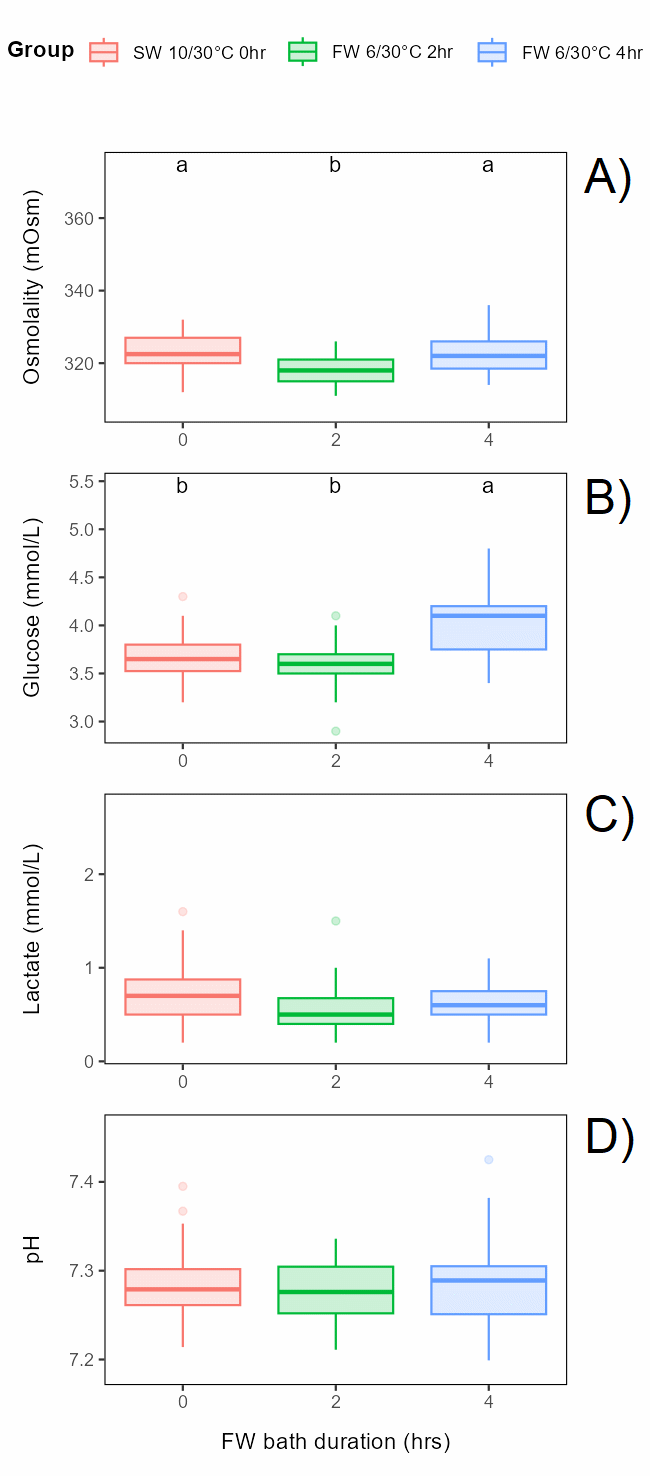
Bath duration (freshwater of 6°C for 0, 2 or 4 h) before exposure to 30°C for 30 s had a small but significant effect on osmolality and glucose, with osmolality lower in the 2 h bath than in the 0 and 4 h bath and glucose level higher in the 4 h bath, while lactate and pH was not affected (Figure 6.4).
Thermal temperature (10, 28, 30 or 32°C) following bath (freshwater of 6°C for 4 h) had a significant effect on osmolality, glucose and lactate, with osmolality lower in all thermal groups than in the 10°C control group, glucose higher in the 30°C group than in the 28°C group, and lactate lower in the 32°C group than in the control group. pH was not affected (Figure 6.5).
It should be noted that all differences, although significant, between all groups (Figure 6.6) were relatively small, and no plasma parameters in any group were off normal levels 3 days after treatment (Noble et al., 2020).
6.2.2 - Welfare scores
Most individuals had clear or sever scale loss in all treatment groups. More than 90% of the individuals were scored at level 2 or 3 for scale loss in most groups. The majority of the individuals had also fin damage of level 2 or 3 most groups. Other welfare indicators were less prevalent (Table 6.2). However, no clear “dose-response” pattern of bath duration, bath temperature, bath salinity or thermal temperature could be seen for any welfare indicator, and we assume that most deviations were present before the treatment. High scale loss levels may be due to relatively high stocking densities in small tanks (10-11 individuals of ~2 kg in 400 L tanks, ~50 kg/m3), and fin erosion, especially on the caudal fin, is common in rainbow trout.
| Bath | Thermal temperature | Scoring level | Scale loss | Skin haemorr. | Body wound | Snout damage | Eye opacity | Eye damage | Opercular damage | Fin damage |
| FW_6°_2h | 30 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 34 |
| 2 | 53 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | ||
| 3 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | ||
| FW_6°_4h | 30 | 1 | 9 | 0 | 0 | 0 | 0 | 0 | 3 | 41 |
| 2 | 78 | 0 | 0 | 6 | 0 | 3 | 3 | 50 | ||
| 3 | 13 | 0 | 0 | 0 | 0 | 0 | 3 | 9 | ||
| SW_10°_0h | 30 | 1 | 48 | 0 | 0 | 9 | 0 | 15 | 0 | 70 |
| 2 | 48 | 0 | 0 | 15 | 0 | 0 | 0 | 30 | ||
| 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SW_6°_4h | 30 | 1 | 6 | 0 | 0 | 3 | 0 | 9 | 3 | 47 |
| 2 | 81 | 0 | 0 | 0 | 0 | 6 | 0 | 53 | ||
| 3 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| FW_10°_4h | 30 | 1 | 10 | 0 | 0 | 6 | 0 | 13 | 3 | 48 |
| 2 | 65 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | ||
| 3 | 26 | 0 | 0 | 0 | 0 | 3 | 0 | 6 | ||
| FW_6°_4h | 28 | 1 | 6 | 0 | 0 | 42 | 3 | 21 | 0 | 33 |
| 2 | 94 | 0 | 0 | 9 | 0 | 12 | 0 | 67 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | ||
| FW_6°_4h | 32 | 1 | 0 | 0 | 0 | 31 | 22 | 41 | 0 | 13 |
| 2 | 100 | 0 | 0 | 3 | 0 | 6 | 0 | 88 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | ||
| FW_6°_4h | 10 | 1 | 6 | 0 | 0 | 25 | 16 | 53 | 0 | 47 |
| 2 | 94 | 0 | 0 | 3 | 0 | 19 | 0 | 53 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SW_10°_4h | 10 | 1 | 12 | 0 | 3 | 9 | 0 | 3 | 0 | 27 |
| 2 | 82 | 0 | 0 | 0 | 0 | 0 | 0 | 70 | ||
| 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
6.3 - Experiment 6: Delousing efficacy
6.3.1 - Louse detachment during bath treatment
Lice loss during bath treatment could only be estimated in the second week when all baths were either freshwater of 6°C for 4 h (9 tanks), or seawater of 10°C for a few minutes (3 tanks). In the freshwater tanks, 37 out of a total of 386 (10%) of the female lice, and 23 out of a total of 471 (5%) male lice, detached during the bath. In seawater tanks these numbers were 3 out of 137 females (2%) and 3 out of 181 males (2%). These lice were not included in the detachment rates during the subsequent thermal treatments (described below).
6.3.2 - Louse detachment during thermal treatment
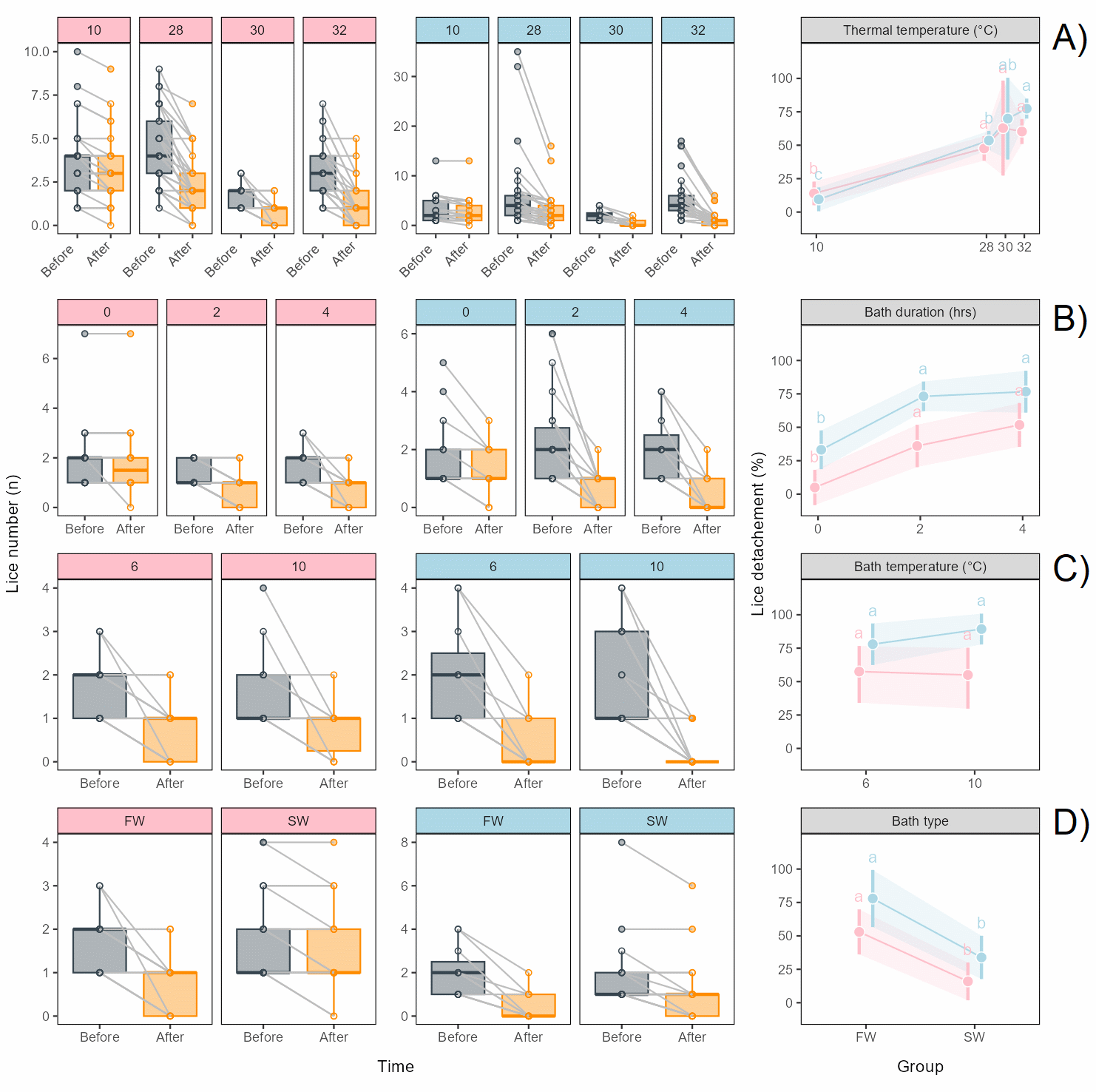
When all bath parameters were kept constant (freshwater, 6°C, 4 h) and the thermal temperature changed, detachment of both female and male lice during thermal exposure was higher for all thermal temperatures than the 10°C control (Figure 6.7A). For females, the effect did however not differ between the thermal temperatures, while for males the effect was higher at 32°C. The delousing effect was slightly higher but with a similar pattern for male lice (Figure 6.7A).
When only bath duration was reduced and bath salinity (freshwater), bath temperature (6°C) and thermal temperature (30°C) kept constant, detachment of both female and male lice during thermal exposure was not significantly different after 4 h bath than after 24 h bath, while with no freshwater bath before thermal exposure (0 h) detachment was significantly lower, with almost no effect on females (Figure 6.7B). The effects on female and male lice showed the same pattern but were generally higher for males (Figure 6.7B).
When only bath temperature was changed and bath salinity (freshwater), bath duration (4 h) and thermal temperature (30°C) kept constant, detachment of both female and male lice during thermal exposure was not significantly different with 10°C bath than with 6°C bath, while the effect was generally higher for males (Figure 6.7C).
When only bath salinity (freshwater or seawater) was changed and bath temperature (6°C), bath duration (4 h) and thermal temperature (30°C) kept constant, detachment of female and male lice during thermal exposure was significantly higher after freshwater bath than after seawater bath. Again, the pattern was similar for males and females but the effect higher for males (Figure 6.7D).
6.3.3 - Delayed louse detachment
The delayed detachment, i.e., during the 3 days period following treatment, of both male and female lice was lowest in the full procedural control, with almost no detachment, and highest in the 32°C thermal group where >half of the lice still attached to the fish immediately after thermal treatment were gone 3 days later. For the remaining treatments there was no clear pattern in how bath duration, bath temperature or bath salinity affected delayed attachment (Table 6.3).
| Bath salinity | Bath temperature (°C) | Bath duration (h) | Thermal temp | Males after thermal treatment | Males on Day 3 | % males delayed detachment | Females after thermal treatment | Females on Day 3 | % females delayed detachment |
| FW | 6 | 4 | 30 | 5 | 3 | 40 | 12 | 9 | 25 |
| FW | 6 | 2 | 30 | 13 | 8 | 38 | 17 | 14 | 18 |
| SW | 6 | 4 | 30 | 23 | 12 | 48 | 32 | 27 | 16 |
| FW | 10 | 4 | 30 | 4 | 3 | 25 | 11 | 10 | 9 |
| FW | 6 | 4 | 10 | 75 | 52 | 31 | 99 | 73 | 26 |
| SW | 10 | 0 | 30 | 17 | 16 | 6 | 43 | 30 | 30 |
| FW | 6 | 4 | 28 | 91 | 51 | 44 | 79 | 61 | 23 |
| FW | 6 | 4 | 32 | 38 | 17 | 55 | 35 | 16 | 54 |
| SW | 10 | 0 | 10 | 176 | 168 | 5 | 131 | 130 | 1 |
6.3.4 - Overall delousing efficacy
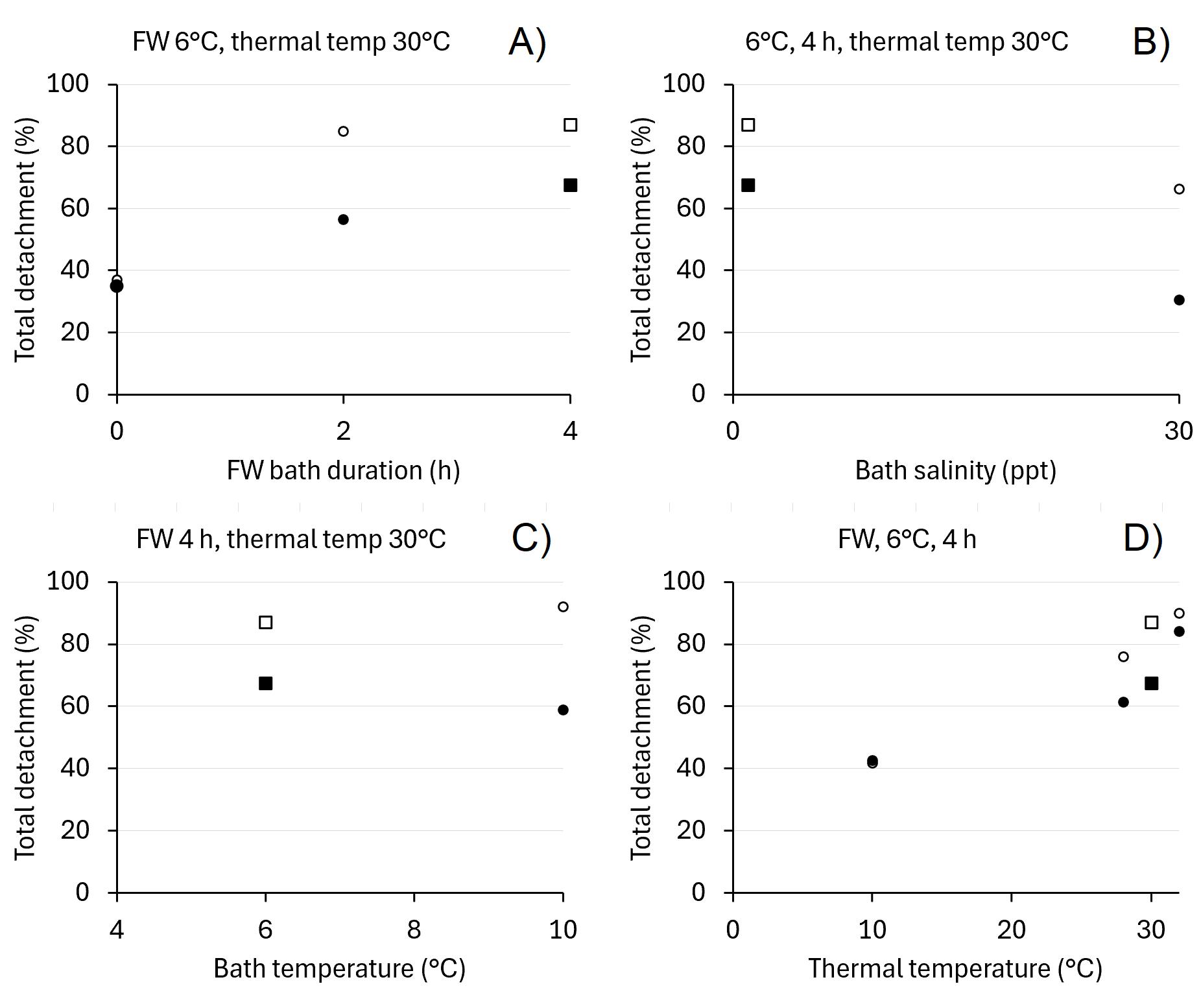
The effects of the different treatments on the overall delousing efficacy (detachment during bath + during thermal treatment + delayed detachment) was not tested statistically due to few replicates (no data at individual level) but showed similar patterns as the detachment during thermal treatment alone, with no or little difference between 2 and 4 h bath duration and between 6 and 10°C bath temperature, higher detachment after freshwater than seawater bath and higher detachment at the higher thermal temperatures (Figure 6.8). With bath detachment and delayed detachment added, the estimated overall effect was of cause higher than the immediate thermal detachment presented in Figure 6.8, but still relatively low, for females 84% as the highest detachment of all treatment combinations (4 h bath at 6°C freshwater followed by 32°C thermal) and only 61% after bath followed by 28°C. Thermal treatment at 30°C without preceding freshwater bath (i.e., 0 h bath duration in Figure 6.8A) gave 35% detachment of females, slightly lower than 4 h freshwater bath without subsequent thermal treatment with 43% (i.e., 10°C thermal temperature in Figure 6.8D).
7 - Delousing efficacy and mortality in commercial settings
Information around delousing events at commercial sites was collected from partners in the project team (see Section 1.2). A registration datasheet was provided to partners that included numerous parameters including details about the site, hatchery and sea transfer, environment, treatment method and procedure, fasting, mortalities, welfare measures, veterinary assessments, and lice counts. This datasheet was adapted from a similar task in the project Welfare Severity (NFR 326980).
Data were delivered sporadically and opportunistically to the project, therefore we were limited in the quantity and range/variety of delousing events that made up the dataset. The initial dataset included ~50 unique delousing events (i.e. delousing that were applied in sequence to a number of cages at the site would be considered a single event) across sites largely within PO4. We removed site identifiers and pooled data so that contributions would remain anonymous. The majority of events were either thermal treatments alone, freshwater bath followed by thermal treatment, or use of a chemotherapeutant.
To complement the project objectives, we investigated the effect of treatment on mortality and louse removal, while exploring whether other factors (such as environment) had an influence on outcome. It was, however, clear that managerial decisions heavily influenced which treatment principle was used. For the collected dataset freshwater treatment followed by thermal treatment tended for instance to be preferred on fish with already-elevated mortality (Fig 7.1A). Percentage point increase in mortality in the 4 weeks after delousing, compared to the 4 weeks before could, however, be partitioned into thermal treatments below 33°C, which again could be partitioned into freshwater followed by thermal and thermal treatment alone vs. chemical treatment (Figure 7.1B). Average percentage point increase in mortality for these partitions was 1.1 for the fish treated at or above 33°C, 1.2 for the fish treated chemically and only 0.045 for the fish treated below 33°C. It should, however, be underlined that these results, due to the many confounding factors of the dataset, must be viewed as particular for this dataset.
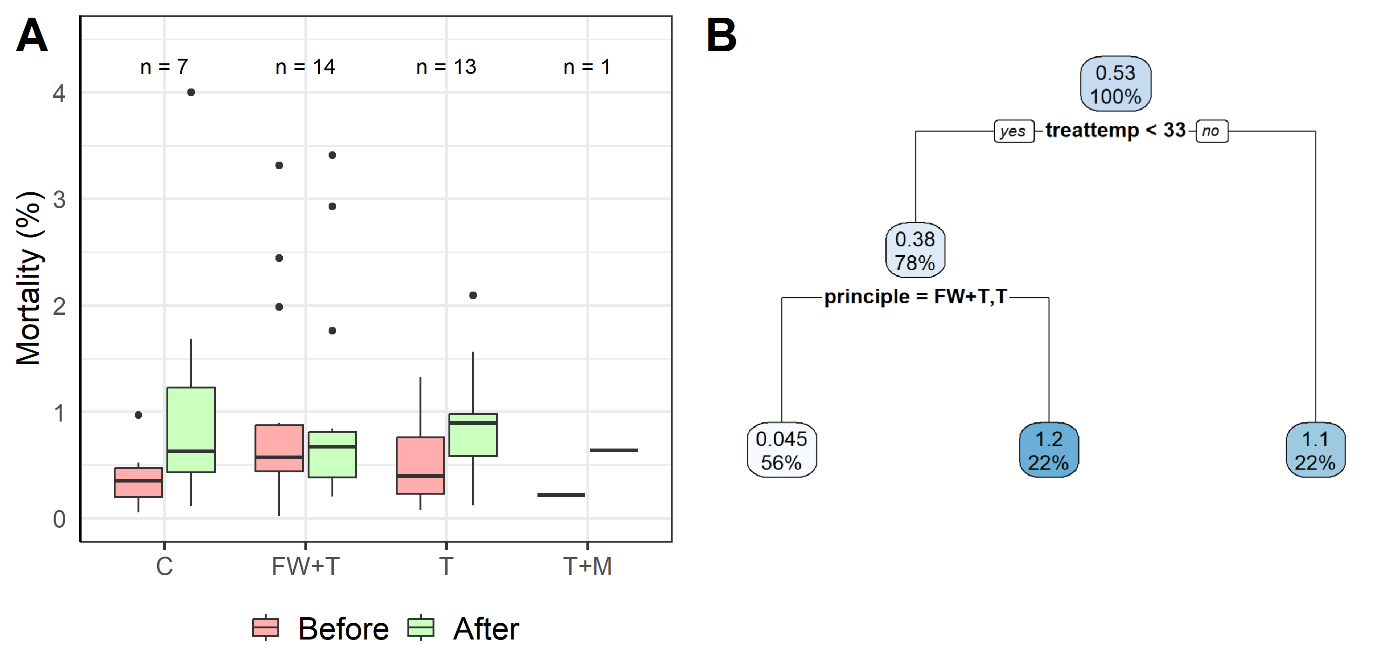
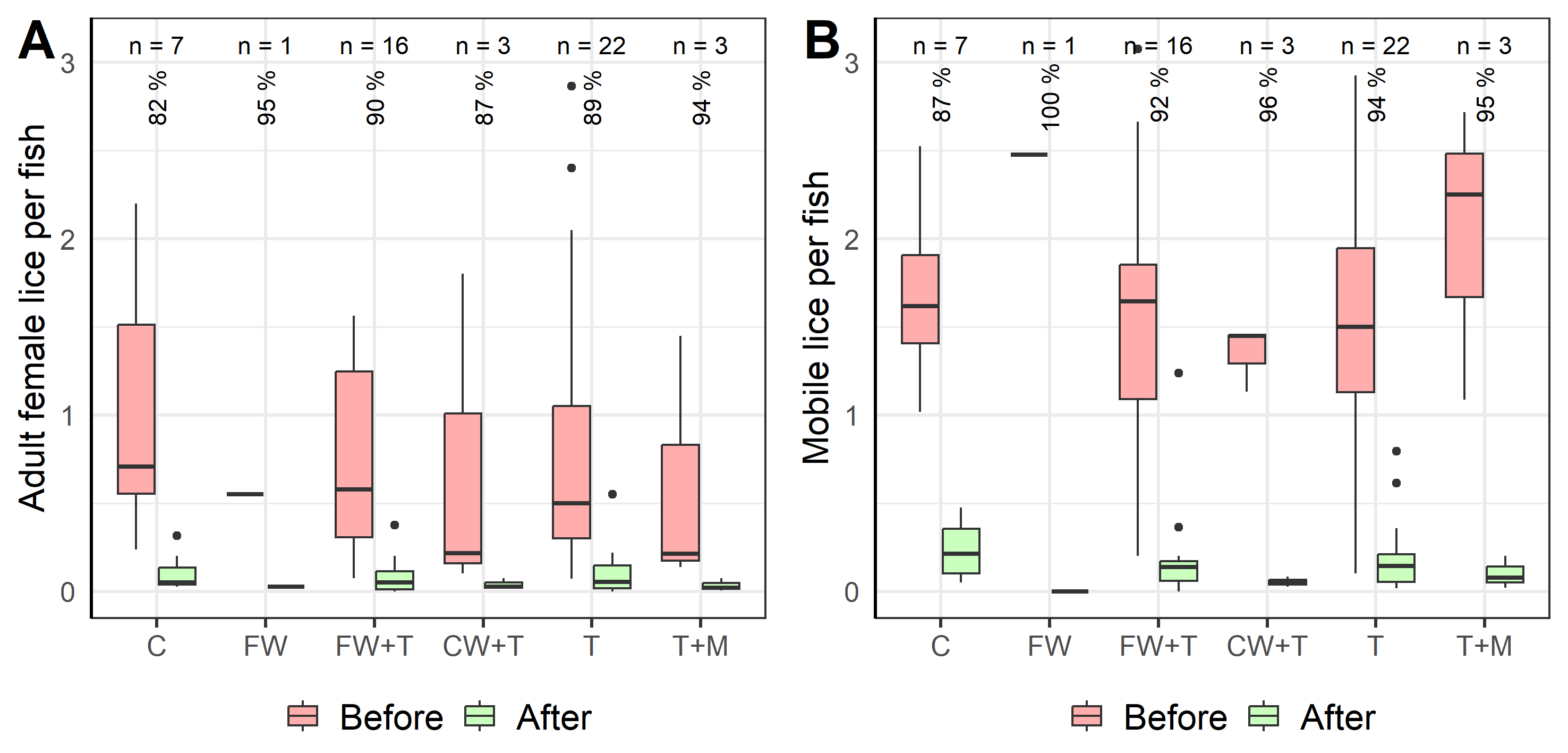
All recorded treatments reported efficient removal of adult female lice (Fig. 7.2A) and mobile lice (Fig. 7.2B), with high proportions of louse removal across treatment types albeit with some examples only having few replicate events. The high removal rate of adult females using freshwater treatments alone (n=1) or in combination with thermal treatment (n=16) was on average 90-95%. This is higher than the removal rates that we found in the tank experiment (Section 6.3, Figures 6.8 and 6.9), highlighting the contrast between research- and commercial-scale practices while adding possible evidence to the hypothesis of increased detachment rate with handling (Bui et al. 2023, Hansen, 2025).
8 - General discussion
This project has provided new, species-specific knowledge on rainbow trout responses to freshwater baths, thermal exposures, and their sequential application for acute control of salmon lice. Unlike Atlantic salmon, rainbow trout showed distinct physiological and behavioural reactions to these treatments, underlining the need for dedicated welfare and efficacy thresholds for this species. The integration of pilot studies, controlled experiments, and commercial data has revealed both opportunities and limitations in applying these methods in practice.
8.1 - Freshwater baths
Freshwater bath before thermal treatment increased delousing efficacy compared to thermal treatment without preceding freshwater bath. However, we found no additional effect of 4 h bath compared to 2 h bath. This suggests that:
-
freshwater weakens the lice, probably due to osmotic challenge, making them detach more easily during the thermal treatment. Increased ΔT° when bathed in 6°C freshwater compared to the control bath of 10°C could be an additional factor, but since we found no difference in efficacy between 6°C and 10°C freshwater bath increased ΔT° does not appear to be the main contribution to the additional effect of thermal treatment preceded by freshwater bath.
-
4 h freshwater bath does not weaken the lice much more than 2 h bath, at least not to give different detachment rates. When attached to a host without mechanical, thermal or other disturbances adult salmon lice can stay attached to their host for several days (Wright et al. 2016), although non-attached lice are more vulnerable to freshwater (Hahnenkamp and Fyhn 1985). Also in our study 4 h freshwater bath had low effect on detachment when it was not followed by thermal treatment. Still, 2 h was here enough to achieve improved detachment when the lice were subsequently exposed to thermal stress. In case chilling down the lice to increase ΔT° for a given thermal temperature have any additional effect, 2 h is far longer than required to chill down the thin lice body and probably also the fish (Pépino et al. 2015).
Freshwater bath of 4 h or more did however affected osmoregulation of both small and large trout, with reduced osmolality. We found no clear effects of temperature of the freshwater bath on physiology. That osmoregulation is affected immediately after freshwater bath is expected, but for small trout osmolality was still affected 3 days after return to seawater. For longer freshwater baths of 6 h or more, also pH was clearly increased after 3 days. Although the decreased osmolality and increased plasma pH were still within tolerance ranges and no mortality or deviant behaviour occurred, freshwater baths, especially with durations of 4 h or more, induce some level of stress, in addition to handling and crowding stress trout will experience in a freshwater treatment in a wellboat.
In our study only detachment of adult lice was evaluated. In a commercial delousing operation additional effects of the freshwater bath, for instance on AGD (Powell et al. 2015) and earlier life stages of lice, including eggs (Wright et al. 2016; Båtnes et al. 2024), may also be of interest, and require longer bath durations. For instance, the proportion of eggs hatching after freshwater bath decline with bath duration, with 30, 16 and 0% hatching after 2, 4 and 6 hours, respectively (Båtnes et al. 2024).
The freshwater used in our experiments were buffered with seawater to ~1 ppt salinity. This could potentially have resulted in lower efficacy than if pure freshwater was used. For free swimming preadult lice survival after 24 h is considerably higher at 5 ppt than at 0 ppt salinity (Andrews and Horsberg 2020). The osmotic stress in freshwater is however higher in free swimming lice than in lice attached to a host that can feed on mucus and body fluids to compensate for the osmotic stress (Hahnenkamp and Fyhn 1985). However, the salinity will usually contain some salt also in a wellboat, as some seawater is loaded with the fish. For instance, Guttu et al. (2024) reported maximum salinities between 0.2 and 1.0 ppt in the wells during a commercial delousing operation.
8.2 - Thermal exposure
Trout displayed strong behavioural responses to thermal exposure, with aversive behaviours and loss of equilibrium (LOE) increasingly common above 30°C. Small trout tolerated acute exposures somewhat better than large trout, where reduced relative heart size and cardiac limitations likely contributed to poorer tolerance in large trout. Cardiac abnormalities and arteriosclerosis with severe reduction in blood flow have been reported in farmed rainbow trout (Brijs et al. 2020) and reduced relative heart size in larger individuals was found in the current project. Physiological parameters (e.g., plasma glucose, lactate) remained within normal ranges three days post-treatment (only analysed for small trout). However, in the experiment with large trout the majority of the individuals in both acclimation temperature groups (6 and 12°C) lost equilibrium at all thermal temperatures (28-34°C), but behavioural welfare indicators highlighted that temperatures ≥32°C are particularly stressful.
Exposure to 34°C resulted in long LOE durations with some individuals being out of equilibrium for more than 8 minutes after they were returned to normal temperature. Data from the Welfare Severity project (NFR 326980) suggest that the loss of equilibrium in thermally exposed (32°C) rainbow trout is associated with heart arrythmia. While cold baths have been claimed to have a sedative effect on trout before thermal treatment, we did not find support for sedative effect of colder water in our study. LOE occurred sooner and lasted longer with 6 than 12°C acclimation temperature, and the intensity of the behavioural response not lower. As discussed above, lower bath temperature before thermal treatment had also no beneficial effect on the delousing efficacy.
8.3 - Combinations versus single treatments
The combination of freshwater bath (4 h at 6°C) followed by thermal treatment (30°C) improved delousing efficacy compared with either method alone. The effect of thermal treatment of 30°C without preceding freshwater bath was surprisingly low with only 4% of the females detached during treatment and an overall detachment (including 2% loss in the “sham” bath and delayed effect after thermal treatment) of 35%. The corresponding numbers when thermal treatment was preceded by freshwater bath was 52% during thermal treatment and an overall detachment of 67%. On the other hand, when freshwater bath was followed by a 10°C “sham” thermal bath detachment of females was also low with 14% during the “thermal” treatment and an overall detachment of 43%. Overall female lice detachment peaked at ~84% under the most effective conditions (freshwater bath+32°C). Thus, sequentially combining freshwater bath with thermal treatment improves efficacy. This was also found in a Hansen’s (2025) masters thesis in this project.
From a welfare perspective, small but significant effects on osmolality and glucose were detected after 3 days, but no severe disturbances. It should be noted that behaviour during the combination treatments was not analysed. These findings suggest that while sequential methods can achieve higher louse removal efficacy, trout respond aversely and thus welfare must be carefully managed if choosing this strategy.
8.4 - Experimental versus commercial delousing
According to the guidelines from the Norwegian Food Safety Authority (Mattilsynet 2022) delousing operations with a removal of <75% of the lice are not suitable and cannot justify the welfare load on the fish, and the goal should be >90%. In our experiments the removal was below the 75% limit in all treatment combinations except the overall detachment with freshwater bath+32°C. Commercial data showed that all recorded treatments reduced adult female and mobile lice levels with >80%. Freshwater–thermal combinations achieved 90–95% removal. The discrepancy between experimental and commercial efficacy treatments is probably due to the much higher mechanical impact in commercial operations, where fish are crowded, pumped and transported in chutes, resulting in much contact with equipment and other fish. A master thesis done in the present project (Hansen 2025) showed that detachment of female lice was higher when the basket in which the trout was treated (Figure 6.1) was repeatedly lowered and elevated in the thermal treatment water then when kept motionless. Bath treatment in a wellboat with the well filled with either freshwater or seawater (as control) showed that a large proportion of the lice loss (31% for females and 51% for total lice loss) in the freshwater treatment was also present in the seawater treatment (Reynolds 2013), demonstrating that much of the effect is attributed to mechanical impact. More recent studies support that much of the effect is due to mechanical impact (Thompson et al. 2023, Guttu et al. 2024).
Mortality during or in the 3 days following treatment did not occur in any of our experiments. Due to the low number of individuals in each treatment group it cannot be concluded that any of the treatments are not associated with increased mortality risk, since low mortality rates are difficult to predict without the use of large groups. For instance, with zero mortality in a group of 20 fish, the 95% confidence interval for mortality is 0-14% (Clopper–Pearson method). Mortality reported after the commercial delousing operations were usually <1% but variable. Here, treatment choice and operational decisions was probably affected by influencing factors like health status of the fish which likely is linked to mortality outcomes. Comparing mortality risk between different methods must be done with great caution. That being said, treatments at ≥33°C or with chemotherapeutants produced higher post-delousing mortality (~1% increase from pre-treatment levels), whereas thermal exposures <33°C were associated with minimal additional mortality (~0.045%). This suggests that temperature thresholds and operational context critically influence welfare outcomes in practice. The different nature of commercial operations compared to controlled tank experiments, like the increased mechanical impact described above, do not only potentially affect delousing efficacy but also welfare risk. The longer duration of the full treatment procedure from crowding to return to the cage due to the large number of individuals also represent an increased welfare load and higher risk.
8.5 - Salmon vs rainbow trout
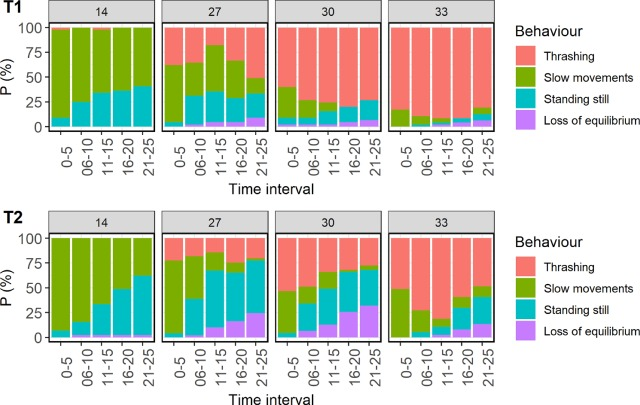
The behavioural response of rainbow trout to thermal temperatures (≥28°C) is generally different from that of salmon, especially for large fish. The majority of the large trout lost equilibrium shortly after introduction to the thermal bath. Salmon usually respond with strong trashing behaviour during most of the exposure duration and lose equilibrium to a much lesser degree, even if it occurs also in salmon (Nilsson et al. 2019; Moltumyr et al. 2022; Bui et al. 2022, Figure 8.1). In previous experiments in the Welfare Severity project loss of equilibrium in large rainbow trout was associated with heart arrythmia, while salmon did not lose equilibrium and no heart arrythmia was detected in salmon (Timmerhaus et al., unpublished data). Hansen (2025) also observed loss of equilibrium during thermal exposure in small (130-300 g) rainbow trout but not in salmon.
In the pilot trial, rainbow trout tolerated salinities up to ~31 ppt without major welfare challenges, but transfer to full-strength seawater (34 ppt) caused a stop in growth and some wound development. In earlier attempts to acclimate rainbow trout to full seawater in this project more severe wound development and increased mortality occurred, resulting in cancelled experiments. This supports the hypothesis that trout has narrower salinity tolerance compared to Atlantic salmon, which has also been reported in previous studies (e.g. Fevolden et al. 1993). Identifying this physiological limit was essential to design the subsequent freshwater and thermal treatment trials without confounding effects of chronic osmotic stress, but still with high enough salinity to not negatively affect salmon lice development. Salinities in open cages is usually lower than 34 ppt, at least in parts of the volume and in fjord sites. Salinity tolerances of rainbow trout should however be studied in more detail if more saline waters, like offshore or submerged cages, are being used for trout farming.
In Hansen’s study delousing efficacy did not differ between rainbow trout and salmon for any of the methods freshwater single (12 h bath), thermal single (28 or 32°C) or combination of freshwater (4 h) and thermal (28 or 32°C). Freshwater bath for 12 h had similarly low effect as the seawater control for both species. An interesting observation to follow up was however that rainbow trout got twice as many lice as salmon when infected in common garden, but not when trout and salmon were infected in separate tanks.
8.6 - Conclusions
The results from the series of experiments from this project underscores the importance of species-specific welfare thresholds for rainbow trout delousing. Practices borrowed from Atlantic salmon cannot be directly applied, as trout physiology and behaviour confer unique vulnerabilities (Fevolden et al. 1993; Brijs et al. 2020). Results from this project will make it easier to balance louse control and trout welfare, by avoiding treatments that are stronger than necessary to effective lice removal (e.g. extended bath durations) or incompatible with good trout welfare (e.g. high thermal temperatures).
Effective removal of lice is significant to maintain the effectiveness of treatments across the industry and mitigation potential mechanisms of resistance in lice populations (Stige et al. 2024). These findings provide a robust evidence base for regulatory approval of freshwater–thermal combination treatments in rainbow trout, supporting more sustainable and welfare-oriented aquaculture practices.
8.7 - Sustainability statement
The degree to which this project contributed to improved sustainability include a number of small-scale steps that reflect the host institute’s commitment to sustainable practices. Firstly, good animal welfare is an important part of the UN’s sustainable development goals, and here we ensured robust experimental design while adhering to the 3R’s of use of experimental animals, optimising the number of animals (and therefore food resources) while minimising unnecessary use of tanks or individual fish. The experiments were conducted at the Matre Research Station (HI) which is experienced with all procedures used in this project, including already having the equipment and facilities required to undertake the experiments (and therefore demanding minimal purchases of new equipment), and having expert competence to streamline efficient resource use. The facility has also implemented an environmental management system which is certified by Miljøfyrtårn, which includes limiting pollution and maximising a circular economy. Lastly, the climate footprint associated with travel was minimised, with largely digital meetings and occasional physical meetings (with the option of digital participation) held in Matre which is somewhat local to many of the project partners.
The results of this project can indirectly contribute to improved and sustainable delousing practices in the industry through a) improved animal welfare, b) reduced environmental footprint, c) more efficient and less frequent delousing events (and thus resource and energy use), d) productivity and food-resource efficiency, and e) social responsibility and regulatory compliance. By documenting species-specific physiological responses, welfare impact and delousing efficacy of single and combined treatments, more informed decisions can be made for management strategies in farmed rainbow trout. If stress, injury and mortalities can be minimised through optimised choice of delousing method (and their parameters, e.g. temperature for thermal exposure), then better welfare outcomes and lower mortality can create efficient and reduced consumption of resources (e.g. through feed loss or product loss) and therefore minimise direct and indirect environmental impacts per kg of fish produced. Considering the scale of rainbow trout aquaculture in Norway, even a small improvement of these factors will have a substantial improvement in the sustainability of industry practices.
9 - Main findings and highlights
-
A pilot trial showed that rainbow trout tolerate brackish water up to ~31 ppt, but full-strength seawater (34 ppt) impairs growth and increases risk of lesions, highlighting a narrower tolerance compared to Atlantic salmon.
-
Exposure to 2 hours of freshwater bath is optimal, while up to 4 hours in freshwater baths are generally tolerable, with longer durations causing persistent osmoregulatory disturbances and welfare risks. Bath temperature (6–12°C) had negligible effect on welfare parameters.
-
With thermal exposure (28-34°C), strong behavioural stress responses become common at ≥32°C, particularly in large trout. Loss of equilibrium occurred at all thermal temperatures but lasted for a longer duration at 34°C. Lower acclimation temperature, and thereby higher ΔT° for the same thermal temperature, made loss of equilibrium occur faster and last longer.
-
Freshwater bath followed by 32°C thermal exposure achieved up to ~84% lice overall removal in controlled trials, with moderate welfare impacts. Seawater bath and/or lower thermal temperatures achieved lower lice removal, while effect of bath temperature (6 or 10°C) did not differ.
-
Factors affecting lice removal were similar among male and female lice, but removal was generally higher in males.
-
Fish size should be accounted for when making decisions on delousing strategy: smaller fish in these experiments tolerated and recovered faster from treatments compared to larger fish, in particular thermal treatment.
Norsk
-
Et pilotforsøk viste at regnbueørret tåler brakkvann opp til ca. 31 ppt, mens fullt sjøvann (34 ppt) hemmer vekst og øker risikoen for sår, noe som viser en snevrere toleranse sammenlignet med atlantisk laks.
-
Eksponering i 2 timer ferskvannsbad er optimalt, mens opp til 4 timer i ferskvannsbad generelt er tolererbart. Lengre varighet fører til vedvarende osmoregulatoriske forstyrrelser og velferdsrisiko. Badetemperatur (6–12°C) hadde ubetydelig effekt på velferdsparametre.
-
Ved termisk eksponering (28–34°C) er sterke atferdsmessige stressresponser vanlige ved ≥32 °C, særlig hos stor regnbueørret. Tap av likevekt forekom ved alle temperaturer, men varte lengre ved 34 °C. Lavere akklimeringstemperatur, og dermed høyere ΔT° ved samme eksponeringstemperatur, førte til at likevektstap inntraff raskere og varte lenger.
-
Ferskvannsbad etterfulgt av termisk eksponering på 32°C fjernet totalt ca. 84 % av lusen i kontrollerte forsøk, med moderate velferdskonsekvenser. Sjøvannsbad og/eller lavere temperaturer ga lavere lusefjerning, mens badtemperatur (6 eller 10°C) ikke spilte noen rolle for avlusningseffekten.
-
Hvilke faktorer som påvirket avlusningseffekt var likt for hann- og hunnlus, men avlusningseffekten var generelt høyere for hannlus.
-
Fiskestørrelse bør tas hensyn til ved valg av avlusingsstrategi: mindre fisk i disse forsøkene tålte behandlingene bedre og kom seg raskere enn større fisk, særlig ved termisk behandling.
10 - References
Andrews M & Horsberg TE (2020) Sensitivity towards low salinity determined by bioassay in the salmon louse, Lepeophtheirus salmonis (Copepoda: Caligidae). Aquaculture. doi: 10.1016/j.aquaculture.2019.734511
Brijs J, et al. (2020) Prevalence and severity of cardiac abnormalities and arteriosclerosis in farmed rainbow trout (Oncorrhynchus mykiss). Aquaculture. doi: 2020.735417.
Bui S, Halttunen E, Mohn AM, Vågseth T, Oppedal F (2018) Salmon lice evasion, susceptibility, retention, and development differs among host salmonid species. ICES Journal of Marine Science. doi: 10.1093/icesjms/fsx222.
Bui S, Madaro A, Nilsson J, Fjelldal PG, Iversen MH, Brinchman MF, Venås B., Schrøder MB, Stien LH (2022) Warm water treatment increased mortality risk in salmon. Vet. Anim. Sci. doi: 10.1016/j.vas.2022.100265.
Bui S , et al. (2023) Optimising delousing strategies: developing best practice recommendations for maximal efficacy and positive welfare – Final report. Rapport fra Havforsningen, 2023-29. https://www.hi.no/en/hi/nettrapporter/rapport-fra-havforskningen-en-2023-29
Båtnes AS, Bjørnland T , Hansen SS, Furberg MH, Middelthon M, Ekundayo OI , Olsen Y, Sæter AF, Bengston JM, Miljeteig C (2024). Ikke-medikamentell kontroll av lus: sammenlikning av avlusingsmetoder på bakgrunn av avlusingseffekt, fiskevelferd, toleranse mot og smittepotensial etter avlusing. NTNU, Taskforce lakselus. Sluttrapport – FHF-prosjektnummer 901688. https://www.fhf.no/prosjekter/prosjektbasen/901688/
Dalvin S, et al. (2020) Rainbow trout Oncorhynchus mykiss skin responses to salmon louse Lepeophtheirus salmonis: From copepodid to adult stage. Fish and Shellfish Immunology. doi: 10.1016/j.fsi.2020.05.014.
Fevolden SE, Refstie T, Gjerde B (1993) Genetic and phenotypic parameters for cortisol and glucose stress response in Atlantic salmon and rainbow trout. Aquaculture,. doi: 10.1016/0044-8486(93)90457-A.
Fevolden SE, Røed KH, Fjalestad K (2003) A combined salt and confinement stress enhances mortality in rainbow trout (Oncorhynchus mykiss) selected for high stress responsiveness. Aquaculture. doi: 10.1016/S0044-8486(02)00131-X
Guttu M, Gaasbø M, Båtnes AS, Olsen Y (2024) The decline in sea lice numbers during freshwater treatments in salmon aquaculture. Aquaculture. doi: 10.1016/j.aquaculture.2023.740131
Hamre LA, Glover KA, Nilsen F (2009) Establishment and characterisation of salmon louse (Lepeophtheirus salmonis (Krøyer 1837)) laboratory strains. Parasitology International. doi:10.1016/j.parint.2009.08.009
Hahnenkamp L, Fyhn HJ (1985). The osmotic response of salmon louse, Lepeophtheirus salmonis (Copepoda: Caligidae), during the transition from sea water to fresh water. Journal of Comparative Physiology B. doi:10.1007/BF00687479
Hansen LT (2025) The effectiveness of freshwater, thermal, and combination delousing methods on Atlantic salmon and rainbow trout. Master thesis, University of Bergen 2025. https://bora.uib.no/bora-xmlui/handle/11250/3207294
Mattilsynet (2022) Veileder om dyrehelsepersonell og bruk av ikke-medikamentelle avlusingsmetoder (IMM). https://www.mattilsynet.no/dyr/dyrehelsepersonell/veileder-om-dyrehelsepersonell-og-bruk-av-ikke-medikamentelle-avlusingsmetoder-imm
Moltumyr L, Nilsson J, Madaro A, Seternes T, Winger FA, Rønnestad I, Stien LH (2022) Long-term welfare effects of repeated warm water treatments on Atlantic salmon (Salmo salar). Aquaculture. doi:10.1016/j.aquaculture.2021.737670
Moltumyr L, Stien LH, Madaro A, Nilsson J (2024) Increasing Dip Net Mesh Size Results in More Fin Splits in Post-Smolt Atlantic Salmon (Salmo salar). Journal of Applied Animal Welfare Science. doi: 10.1080/10888705.2022.2152689.
Nilsson J, Moltumyr L, Madaro A, Kristiansen TS, Gåsnes SK, Mejdel, CM, Gismervik K, Stien LH (2019) Sudden exposure to warm water causes instant behavioural responses indicative of nociception or pain in Atlantic salmon. Veterinary and Animal Science. doi: 10.1016/j.vas.2019.100076
Nilsson J, et al. (2022) Laksvel-Standardisert operasjonell velferdsovervåking for laks i matfiskanlegg. Rapport fra havforskningen, 2022-14. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-2022-14
Nilsson J, et al. (2023) Effect of water temperature and exposure duration on detachment rate of salmon lice (Lepeophtheirus salmonis); testing the relevant thermal spectrum used for delousing. Aquaculture, doi: 10.1016/j.aquaculture.2022.738879.
Noble C, Gismervik K, Iversen MH, Kolarevic J, Nilsson J, Stien LH, Turnbull JF (Eds.) (2020) Welfare Indicators for farmed rainbow trout: tools for assessing fish welfare 310 pp. ISBN 978-82-8296-620-7.
Overton K, et al. (2019) Salmon lice treatments and salmon mortality in Norwegian aquaculture: a review. Reviews in Aquaculture. doi: 10.1111/RAQ.12299.
Pépino M, Goyer K, Magnan P (2015). Heat transfer in fish: are short excursions between habitats a thermoregulatory behaviour to exploit resources in an unfavourable thermal environment? Journal of Experimental Biology. doi:10.1242/jeb.126466
Powell MD, Reynolds P, Kristensen T (2015) Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquaculture. doi:10.1016/j.aquaculture.2015.05.027
Reynolds P (2013) Ferskvannsavlusning i brønnbåt. Gildeskål Forskningsstasjon AS. DOI: 10.13140/RG.2.1.2371.0563
Rørvik KA, Skjervold PO, Fjæra SO, Steien SH (2000) Distended, water-filled stomach in seawater farmed rainbow trout, Oncorhynchus mykiss (Walbaum), provoked experimentally by osmoregulatory stress. Journal of Fish Diseases. doi: 10.1046/j.1365-2761.2000.00200.x.
Stige LC, Huseby RB, Helgesen KO, Aldrin M, Qviller L (2024) Consequences of reduced effectiveness of salmon lice treatments for lice control. Preventive Veterinary Medicine. doi:10.1016/j.prevetmed.2024.106134
Thompson C, Madaro A, Oppedal F, Stien LH, Bui S (2023) Delousing efficacy and physiological impacts on salmon of freshwater and hyposaline bath treatments. Rapport fra Havforskningen, 2023-11.
Wright DW, Oppedal F, Dempster T (2016) Early stage sea lice recruits on Atlantic salmon are freshwater sensitive. J Fish Dis. doi: 10.1111/jfd.12452
11 - Project deliverables
Conference presentations
-
Nilsson J (2024) Hjertevarme hos regnbueørret – hva skjer når den avluses i varmt vann. Fiskevelferdsseminar: Haltende helse gir ingen lakselykke; Oslo, 2024-09-23.
-
Nilsson J, Myklatun LE, Vågseth T, Fraser T, Bui S, Madaro A (2025) Regnbueørret mister likevekten under termisk eksponering. Frisk Fisk, Tromsø, 11-12 mars 2025.
Popular science articles
-
Nilsson J, Bui S, Vågseth T, Fraser TWK, Timmerhaus G, Stien LH, Madaro A (2025) Regnbueørret responderer ikke som en laks under termisk behandling. Norsk Fiskeoppdrett nr 3 2025, 30-32.
-
Nilsson J, Bui S, Vågseth T, Fraser TWK, Stien LH, Madaro A (2025) Avlusningseffekter av kombinasjonsbehandlinger av regnbueørret. Norsk Fiskeoppdrett nr 10 2025, 50-52.
Master thesis
-
Hansen LT (2025) The effectiveness of freshwater, thermal, and combination delousing methods on Atlantic salmon and rainbow trout. Master thesis, University of Bergen 2025. https://bora.uib.no/bora-xmlui/handle/11250/3207294
Reference group meetings: 7 September 2023; 2 May 2024; 27 Nov 2024; 22 Oct 2025
Periodic reports to FHF: 31 Jan 2024; 1 Jul 2024; 29 Nov 2024; 25 Jun 2025